Aromatic Reactions: Diazotization of Aniline (HNO₂/H₂SO₄)
Diazotization converts anilines (Ar–NH₂) into aryl diazonium salts that become Sandmeyer, Schiemann, or azo intermediates. Nitrous acid is generated in situ from NaNO₂ and cold sulfuric acid, protonated to H₂NO₂⁺, and dehydrated to nitrosyl cation (NO⁺)—that electrophile, not HNO₂ itself, is what the amine attacks. Nitrosation gives an N-nitrosamine that tautomerizes to the diazohydroxide, and proton-assisted dehydration removes water to furnish Ar–N₂⁺ HSO₄⁻. Everything stays between 0 and 5 °C to prevent the diazonium from hydrolyzing to phenol or ejecting N₂ prematurely.
Key Emphasis (Teaching Pivots)
- Electrophile clarity: Nitrosyl cation (NO⁺), generated from HNO₂ + H₂SO₄, is the species attacked by aniline—never draw direct attack on neutral HNO₂.
- Sequence discipline: NO⁺ formation → N-nitrosation (diazohydroxide) → protonation/dehydration → cold storage of Ar–N₂⁺. Skipping a frame hides a mechanistic requirement.
- Temperature lock: Keep everything at 0–5 °C. Warming accelerates hydrolysis to phenol or uncontrolled N₂ loss before the diazonium is captured.
- Amine-class logic: Primary aryl amines diazotize. Secondary amines stall as nitrosamines, tertiary amines merely protonate—call those outcomes explicitly on exams.
- Downstream mindset: Arenediazonium salts are transient reagents. Plan the follow-up (Sandmeyer, Schiemann, azo coupling, hydrolysis) before diazotizing.
Quick Summary
- Reagents/conditions: NaNO₂ + H₂SO₄ (aq, cold) → HNO₂ → NO⁺; strict 0–5 °C; counter-ion is typically HSO₄⁻.
- Outcome: Aniline (or another primary aryl amine) converts to the corresponding arenediazonium salt (Ar–N₂⁺ HSO₄⁻) ready for Sandmeyer, Schiemann, or azo chemistry.
- Class rules: Primary aryl amines succeed; secondary amines form nitrosamines; tertiary amines do not generate diazonium; aliphatic diazonium ions collapse even cold.
- Notes: Nitrosyl formation must precede nitrosation; proton shuttles deliver the diazohydroxide; protonation plus dehydration release water and lock in Ar–N₂⁺.
Mechanism — Diazotization of Aniline (8 Frames)
Mechanistic Checklist
- Make NO⁺ explicitly (HNO₂ + H₂SO₄ → H₂NO₂⁺ → NO⁺ + H₂O); do not shortcut to “HNO₂ attacks.”
- Show the nitrosation event on nitrogen, followed by the diazohydroxide tautomer.
- Protonate the –N=NOH fragment and remove water to reveal Ar–N₂⁺.
- Keep the counter-ion (HSO₄⁻ here) and the temperature callout visible.
- Mention that secondary amines stop as nitrosamines and tertiary amines do not diazotize.
Worked Examples
1. Aniline → Benzenediazonium Hydrogen Sulfate
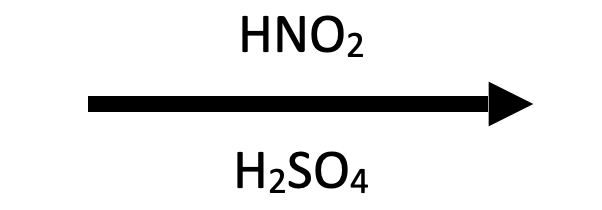
Classic diazotization: cold NaNO₂/H₂SO₄ converts aniline to benzenediazonium hydrogen sulfate, ready for Sandmeyer halogenation or azo coupling.
2. p-Toluidine → p-Methylbenzenediazonium

Electron donation from the para methyl group accelerates nitrosation; the diazonium is still only stable cold and should be captured immediately.
3. m-Methoxyaniline → m-Methoxybenzenediazonium

Even meta-directing substrates diazotize. Keep them cold, then hand the diazonium to Sandmeyer copper halides, Schiemann (HBF₄), or azo coupling partners.
Scope & Limitations
- Works: Primary aryl amines (anilines, heteroaryl analogs) with at least one aromatic carbon bearing the amine.
- Doesn’t: Aliphatic primary amines (diazonium collapses), secondary amines (stop at nitrosamines), tertiary amines (no diazonium).
- Functional sensitivity: Strong acid is present; protect acid-labile functionality or switch to HCl/BF₄⁻ variants if needed.
- Operational: Prepare HNO₂ in situ, maintain 0–5 °C throughout nitrite addition and hold until the follow-up reaction begins.
Edge Cases & Exam Traps
- Phenol formation: Hydrolysis to phenol happens when aqueous mixtures warm—do not attribute phenol to the diazotization step itself.
- Counter-ion swapping: Cl⁻ or BF₄⁻ exchanges are common to improve handling; they are not different mechanisms.
- Over-nitrosation/azo coupling: Strongly activated rings can self-couple if you linger warm; keep cold or capture immediately.
- Router logic: Secondary amines lead to nitrosamines, tertiary amines only protonate—state that explicitly when rejecting substrates.
Practical Tips
- Add cold NaNO₂ solution slowly into the pre-chilled anilinium sulfuric acid mixture with vigorous stirring.
- Use an ice–salt bath or refrigerated jacket to keep 0–5 °C; verify a slight excess of nitrosating agent with starch–iodide paper at the end.
- Charge the nucleophile/catalyst (CuX, HBF₄, activated ring partner) before warming so the diazonium is captured as soon as temperature rises.
- Keep everything in solution—dry arenediazonium salts are shock-sensitive, even as BF₄⁻.
Exam-Style Summary
HNO₂/H₂SO₄ (0–5 °C) protonates nitrous acid to H₂NO₂⁺, loses water to NO⁺, and nitrosates aniline at nitrogen. Proton transfers yield the diazohydroxide, protonation of –N=NOH activates water as a leaving group, and dehydration produces Ar–N₂⁺ paired with HSO₄⁻. Only primary aryl amines diazotize; warming leads to phenols or N₂ loss.
Interactive Toolbox
- Use Mechanism Solver to animate each of the eight diazotization frames (NO⁺ formation, nitrosation, proton shuttles, dehydration prep, and cold storage) with temperature warnings baked in.
- Use Reaction Solver to send the arenediazonium into Sandmeyer, Schiemann, hydrolysis, or azo-coupling branches and see router warnings for secondary/tertiary amines.
- Use IUPAC Namer to confirm names for benzenediazonium salts and their downstream Sandmeyer or phenol products (still no SMILES in the learner-facing text).