Aromatic Reactions: Nitration
Benzene molecules react with nitric acid (HNO3) and sulfuric acid (H2SO4) to form nitrobenzene molecules. The nitration of benzene is a fundamental reaction in organic chemistry, used to introduce a nitro group (-NO₂) into the benzene ring, forming nitrobenzene. This reaction is a classic example of electrophilic aromatic substitution, where an electrophile replaces a hydrogen atom on the aromatic ring:
Nitration Electron Withdrawing Group (EWG)
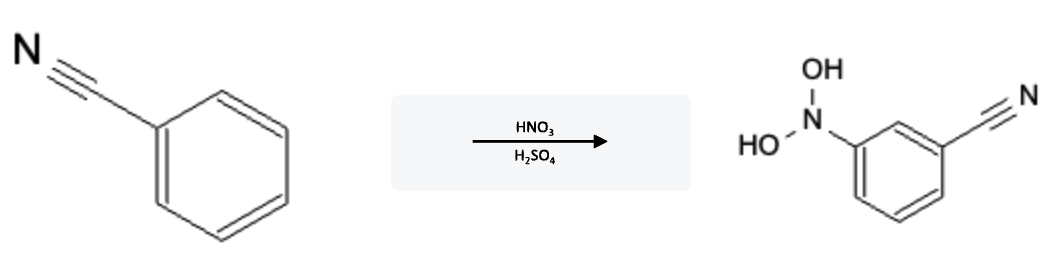
Nitration Electron Donating Group (EDG)
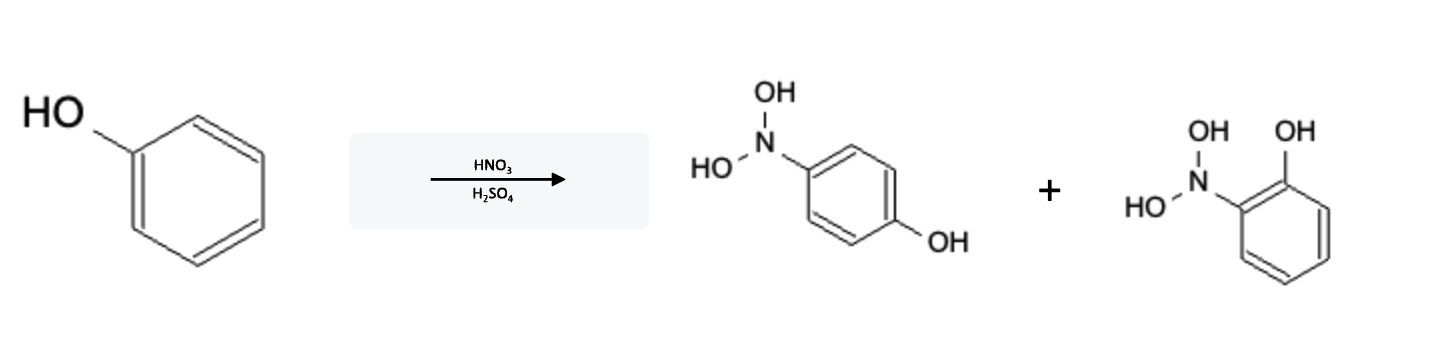
Mechanism
The reaction mechanism is depicted below:
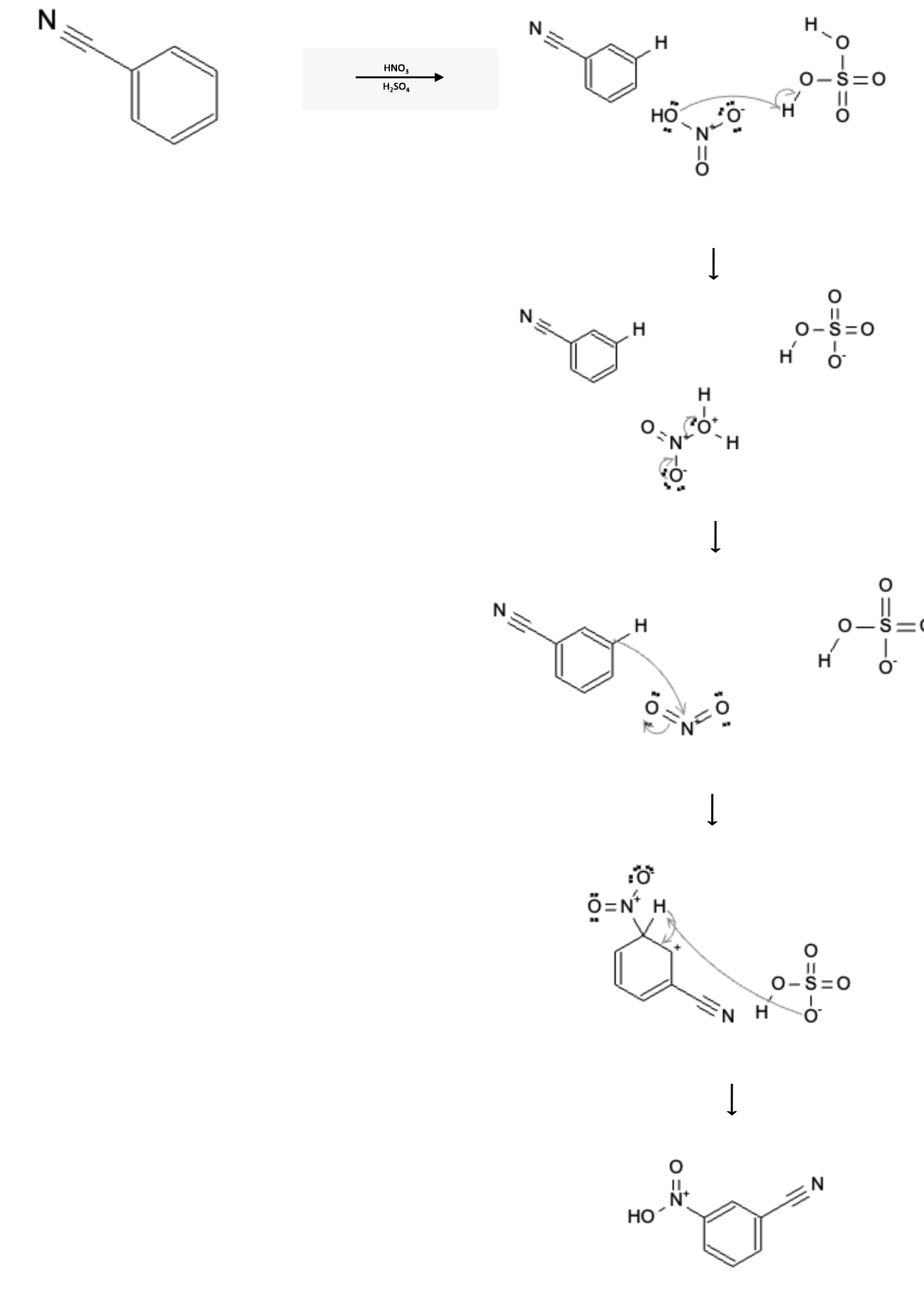
In the first step, the HNO3 molecule is protanated by the H2SO4 acid, leading to the formation of H2NO3.
In the second step, the now protonated oxygen atom on H2NO3 is removed as H2O and leading to the formation of NO2 (nitrogen dioxide).
In the third step, the pi bond from the benzene ring attacks the N atom of nitrogen dioxide, shifting the double bond pi electrons to the O atom of the nitrogen dioxide
In the fourth and final step, the deprotonated HSO4- attacks the hydrogen atom on the benzene ring, sending the hydrogen bond electrons back to the benzene ring, forming the nitrobenzene molecule.
If you have ever watched cartoons and seen a character us TNT, that stands for trinitrotoluene. To make TNT, simply start with toluene and perform this nitration reaction but be careful or you'll end up like Wile E. Coyote!
Practice this reaction using our Reaction Solver!