Aromatic Reductions: Nitrobenzene → Aniline (H₂/Pd, Pt, or Ni)
Catalytic hydrogenation of nitroarenes is a cornerstone transformation in undergraduate organic chemistry. Under hydrogen gas with Pd/C, Pt/C, or Raney Ni, the nitro group adsorbs to the metal surface, is reduced stepwise (–NO₂ → –NO → –NHOH), and leaves as an aniline while water carries away the oxygen atoms. Compared with Béchamp reductions (Fe/Zn + acid), the hydrogenation route needs no acid workup unless you deliberately run in acidic media. Solvents such as EtOH, EtOAc, or AcOH keep the catalyst wetted; the amine can be isolated as the free base or as anilinium when acids are present.
Key Emphasis (Teaching Pivots)
- Surface sequence: H₂ → 2 M–H, nitrobenzene adsorbs, then passes nitro → nitroso → N‑phenylhydroxylamine → aniline.
- Condensation side-path: Ar–NO + Ar–NHOH can condense to azoxy/azo/hydrazo species; under H₂ they hydrogenate onward but mention the possibility.
- Chemoselectivity: Nitro groups usually reduce first, yet Pd/C (and Raney Ni) will also hydrogenate alkenes, alkynes, and can hydrogenolyze Ar–X (Br/I).
- Workup logic: Product emerges as neutral aniline unless the solution is acidic (then isolate anilinium). No NaOH liberating step is obligatory.
Quick Summary
- Reagents/conditions: H₂ (balloon to a few atm) with Pd/C, Pt/C, or Raney Ni; EtOH/EtOAc/AcOH; rt to ~50 °C.
- Outcome: Nitrobenzene → aniline + 2 H₂O (free base, or anilinium under acid).
- Selectivity cautions: Pd/C can dehalogenate Ar–Br/I and hydrogenate C=C/C≡C; Raney Ni at high P–T may over‑reduce the ring to cyclohexylamine.
- Contrast to Béchamp: No Fe/Zn salts, no mandatory NaOH workup—just filter off catalyst and concentrate.
Mechanism — Heterogeneous Hydrogenation (5 Frames)
Mechanistic Checklist
- Depict H₂ → 2 M–H, nitrobenzene adsorption, and sequential hydrogen deliveries (–NO₂ → –NO → –NHOH → –NH₂).
- Mention azoxy/azo/hydrazo condensation as a side-path that is further reduced under H₂.
- Highlight potential hydrogenolysis (Ar–Br/I) and alkene reductions as chemoselectivity risks.
- Product is aniline (or anilinium if acidic solvent); no Fe/Zn salts or NaOH liberations.
Worked Examples
1. Nitrobenzene → Aniline (Pd/C, 1 atm H₂)
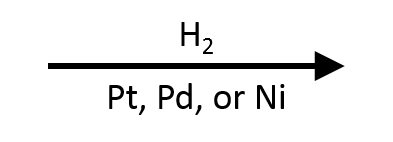
Classic lab-scale reduction: pre-wet Pd/C, apply a balloon of H₂, stir at rt–40 °C, filter the catalyst while wet, and concentrate to obtain aniline.
2. p-Nitrotoluene → p-Toluidine (Raney Ni, 1–3 atm)
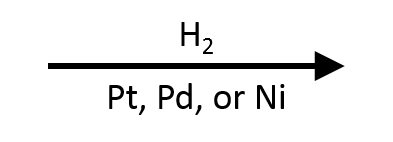
Raney Ni (Ni/Al alloy) at 1–3 atm H₂ and 25–50 °C reduces the nitro group cleanly while the benzylic methyl survives. Avoid higher pressure/temperature if you must preserve the aromatic ring; keep Raney Ni wet because the catalyst is pyrophoric when dry.
3. m-Nitrochlorobenzene → m-Chloroaniline (Pt/C)
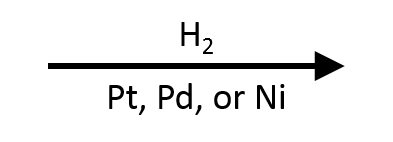
Pt/C keeps aryl chlorides intact more reliably than Pd/C. Use balloon H₂ in EtOH or AcOH and filter promptly. Choose Pt or Ni when halogen retention matters; Pd/C frequently hydrogenolyzes Ar–Br/I under similar conditions.
Scope & Limitations
- Works well: Mono- and polynitroarenes; dinitros give diamines with sufficient H₂/catalyst.
- Sensitive groups: C=C and C≡C hydrogenate readily; benzylic protecting groups or Ar–Br/I can hydrogenolyze (especially on Pd/C).
- Catalyst choice: Pd/C is general but less tolerant of halogens; Pt/C similar activity with better halogen retention; Raney Ni is inexpensive/robust but more prone to over-reduce aromatic rings at elevated P–T.
- Catalyst poisons: Sulfur- or amine-rich substrates can poison catalysts—pre-clean or choose alternative routes.
Edge Cases & Exam Traps
- Reporting an amide or other product: hydrogenation stops at the amine unless a subsequent acylation occurs.
- Ignoring double-bond reduction: alkenyl nitroarenes can hydrogenate across C=C faster than –NO₂ on some catalysts.
- Forgetting hydrogenolysis: Pd/C often cleaves Ar–Br/I; choose Pt/Ni or non-hydrogen conditions to retain halogens.
Practical Tips
- Keep catalysts wet (Pd/C, Pt/C, Raney Ni are pyrophoric when dry); filter through celite while damp and quench residues properly.
- Degas solvents, purge apparatus with N₂, then charge H₂; monitor exotherm as the reduction releases heat.
- If the product is needed as the free base, basify acidic filtrates and extract; otherwise, isolate as anilinium salt directly.
- Remove trace peroxides from EtOAc/EtOH—they can ignite Pd/C during filtration.
Exam-Style Summary
H₂ over Pd/C, Pt/C, or Raney Ni reduces nitrobenzene on the catalyst surface via nitroso and N‑phenylhydroxylamine intermediates to aniline + H₂O. Watch for alkene hydrogenation and Pd/Ni hydrogenolysis of Ar–Br/I; Raney Ni at high pressure/temperature may over-reduce the aromatic ring.
Interactive Toolbox
- Mechanism Solver — Mechanism Solver animates H₂ adsorption, nitro adsorption, nitroso formation, hydroxylamine buildup, and N–O hydrogenolysis/desorption with catalyst overlays.
- Reaction Solver — Reaction Solver compares Pd/C, Pt/C, and Raney Ni on substituted nitroarenes and flags competing alkene or Ar–X reductions.
- IUPAC Namer — IUPAC Namer lets you practice naming nitrobenzene starting materials and the aniline products generated under catalytic hydrogenation.