Azide Reactions: Amine formation from Azides using Triphenylphosphine (Staudinger Reaction)
Azides (N=N=N) react with triphenylphosphine and water to form amines. This reaction is known as the Staudinger Reaction and it is a significant organic transformation that allows the synthesis of iminophosphoranes from azides. Named after Hermann Staudinger who discovered it in 1919, this reaction plays a crucial role in organic synthesis, particularly in the preparation of primary amines:
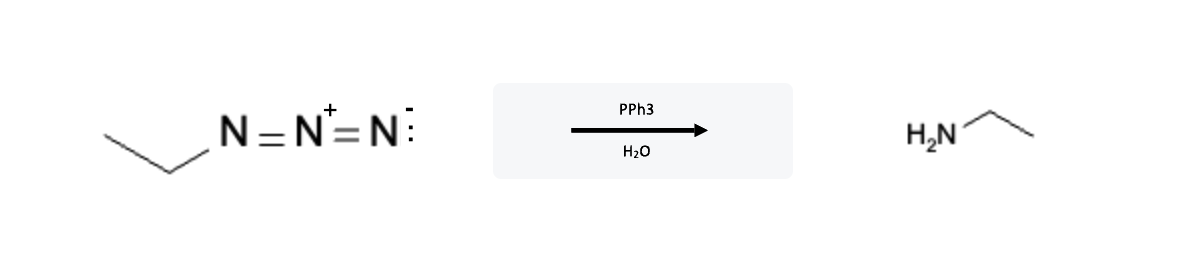
Since this reaction always begins with an azide, the product will always be a primary amine.
Mechanism
The reaction mechanism is depicted below:
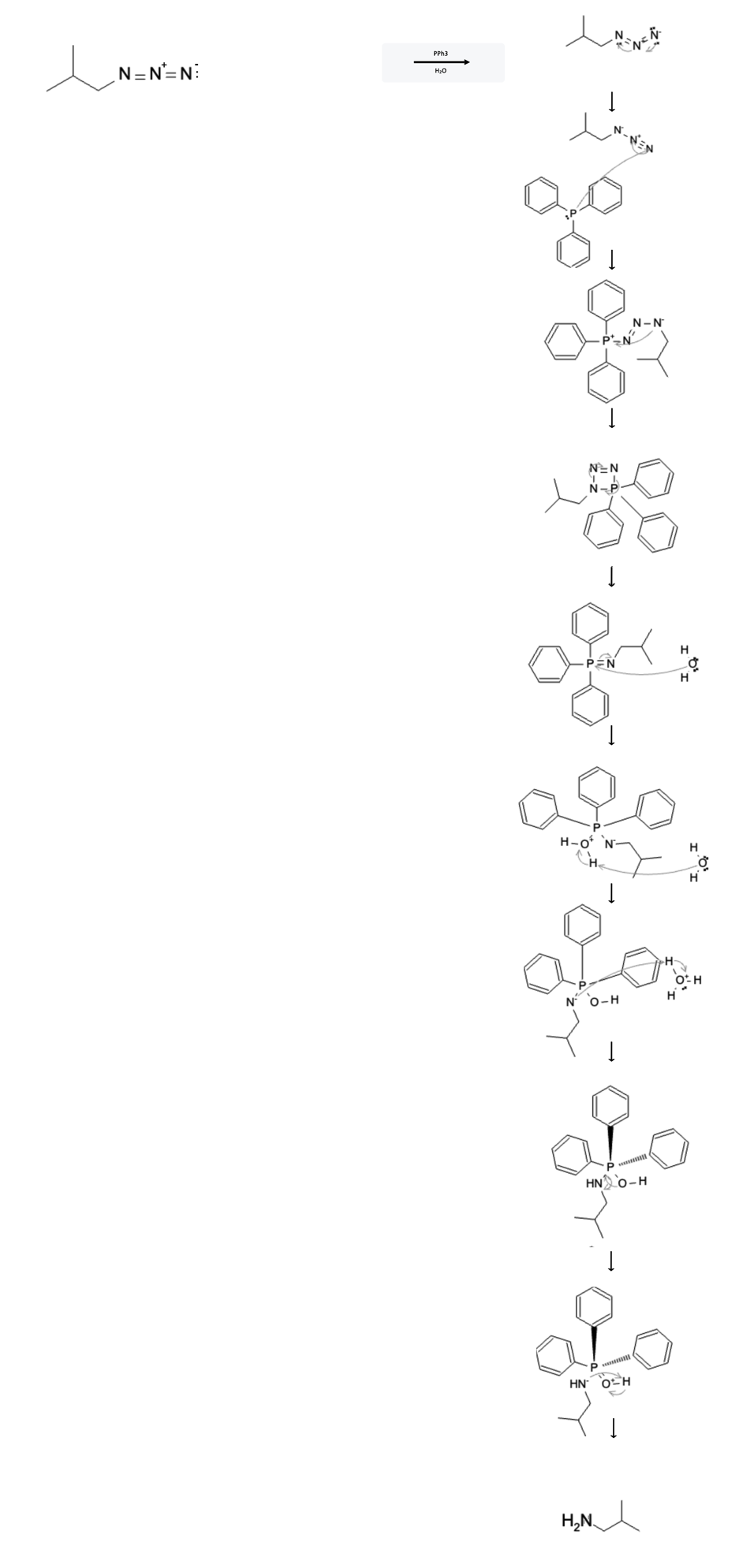
In the first step, the azide bonds rearrange to form a single and triple bond, preparing the molecule for the next step.
In the second step, the triphenylphosphine's phosphorus atom free electrons attack the terminal nitrogen, shifting the pi electrons from the triple bond to the middle nitrogen, now forming a double bond.
In the third step, the negatively charged nitrogen from the azide moiety attacks the phosphorus atom, leading to the formation of a 4-membered ring structure.
In the fourth step, the bonds rearrange and cleave the two outermost nitrogens from the original azide moiety, leaving the most-inner nitrogen still attached to the phosphorus with a newly formed double bond.
In the fifth step, a water molecule attacks the phosphorus, allowing the pi electrons from the phosphorus-nitrogen double bond to go to the nitrogen atom.
In the sixth step, another water molecule deprotantes the newly attached water molecule leading to the formation of an H3O+ molecule.
In the seventh step, the electrons from the negatively charged nitrogen attack the H3O+ molecule, pulling off the extra hydrogen atom.
In the eigth step, the electrons on the oxygen atom come down and form a double bond with the phosphorus atom, which forces the phosphorus-nitrogen bond to break sending the electrons to the nitrogen.
In the ninth step, the newly cleaved, negatively charged nitrogen atom attacks the hydrogen atom attached to the positively-charged, double-bonded oxygen atom completing the reaction. The result is the newly formed amine (as well as triphenylphosphine oxide)
Practice this reaction using our Reaction Solver!