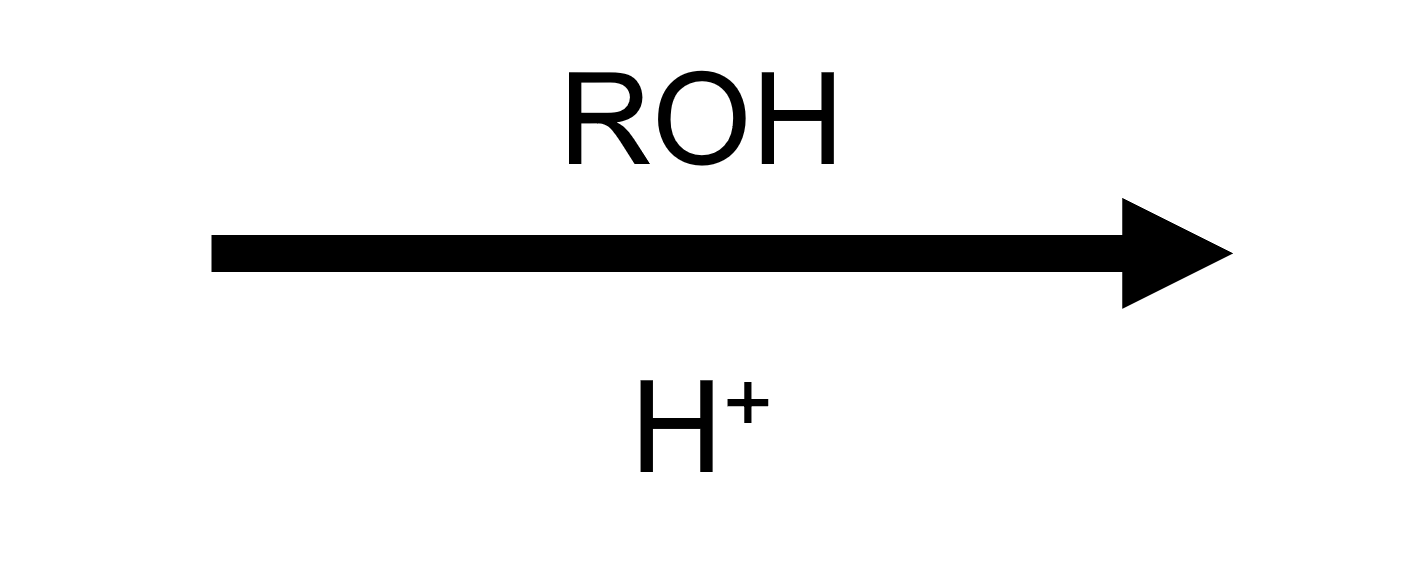
Carboxylic Acids + Alcohols → Esters (Fischer Esterification)
Under strong Brønsted acid catalysis (H₂SO₄, HCl, p‑TsOH) a carboxylic acid and an alcohol equilibrate to an ester plus water. Every step is reversible: you must remove water (Dean–Stark trap, azeotropes, molecular sieves) or flood the reaction with alcohol to favor the ester. Mechanistically the carbonyl oxygen is protonated, ROH adds to form a tetrahedral oxonium, proton transfers convert an OH into water, and elimination of H₂O followed by deprotonation regenerates the acid catalyst.
Quick Summary
| Reagents / conditions | Carboxylic acid + ROH + catalytic H₂SO₄ / HCl / p‑TsOH, heat (reflux in ROH or toluene/Dean–Stark); molecular sieves optional to capture water. |
|---|---|
| Outcome | RCO₂H + R′OH ⇌ RCO₂R′ + H₂O (ester plus water; acid catalyst is regenerated). |
| Mechanism | (1) Protonate carbonyl → (2) ROH attack → (3) water deprotonates the new OR → (4) protonate the original OH → (5) collapse ejects H₂O → (6) deprotonation releases the ester. |
| Equilibrium control | Every step is reversible; removing H₂O or using large ROH excess pushes Le Châtelier’s balance toward the ester. |
| Stereochemistry | No inversion at the acyl center (sp² → tetrahedral → sp²). Intramolecular cases lock in ring sizes (lactones). |
| Contrasts & pitfalls | Phenols/tertiary ROH are sluggish; base quenches the catalyst; if water is not removed, hydrolysis dominates. |
Mechanism — Seven RDKit Steps
Mechanistic Checklist (Exam Focus)
- Protonate the carbonyl oxygen before ROH attacks—no SN1/E1 at an acyl carbon.
- Draw the tetrahedral intermediate explicitly; do not skip straight to ester formation.
- Include proton shuttles that turn the original –OH into H₂O and neutralize the new OR group.
- Water elimination produces a protonated ester; only then is the catalyst regenerated.
- Emphasize reversibility and Le Châtelier strategies (excess ROH, Dean–Stark, sieves).
- Note that phenols / tertiary ROH are sluggish; intramolecular hydroxy acids can cyclize to lactones.
Worked Examples
Benzoic acid + methanol → methyl benzoate
Reactants
Reagents
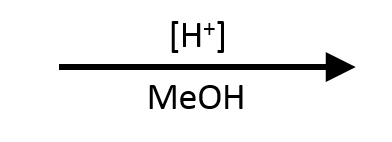
MeOH (solvent), cat. H₂SO₄, reflux, −H₂O
Product
Seafoam highlight traces the methoxy fragment donated by MeOH.
Cyclohexanecarboxylic acid + ethanol → ethyl cyclohexanecarboxylate
Reactants
Reagents
EtOH + p‑TsOH, toluene, Dean–Stark trap removes H₂O.
Product
Highlight shows the ethoxy fragment that replaces the original OH.
γ-Hydroxybutanoic acid → γ-butyrolactone (intramolecular)
Reactant
Reagents

Cat. H₂SO₄, heat; water is distilled off to favor lactonization.
Product
Highlight traces the tethered –OH fragment that cyclized to form the lactone.
Scope & Limitations
- Great matches: Primary/secondary alcohols, unhindered carboxylic acids, hydroxy acids (lactones), diols + diacids (polyesters when water is continuously removed).
- Sluggish partners: Phenols (poor nucleophiles), tertiary alcohols (can dehydrate), very hindered acids.
- Functional-group caution: Acid-sensitive moieties (acetals, tert-butylic groups) may hydrolyze; use milder coupling (e.g., DCC/DMAP) if needed.
- Equilibrium awareness: Without water removal or ROH excess the mixture drifts back to the starting acid + alcohol.
- Transesterification: The same mechanism applies to ester exchange—direction depends on which component is in excess.
Practical Tips
- Use the alcohol as solvent when possible; otherwise reflux in toluene/benzene with a Dean–Stark trap.
- Add a drying aid (3 Å sieves) if a Dean–Stark setup is impractical—removing every drop of water matters.
- Choose the catalyst intentionally: p‑TsOH (solid, non-volatile) for organic media; HCl(g) in MeOH for fast methylations; sulfuric acid for classic textbook setups.
- Neutralize carefully at workup (NaHCO₃ wash) before concentrating to avoid acid-catalyzed rearrangements.
- Intramolecular cases benefit from dilute conditions so the tethered –OH outcompetes intermolecular ROH.
Exam-Style Summary
RCO₂H + R′OH —(cat. H⁺, heat; −H₂O or excess ROH)⇌ protonated carbonyl → ROH addition → proton shuttles (make H₂O) → collapse ejects water → deprotonation → RCO₂R′.
Always mention how you push the equilibrium (remove water, use ROH as solvent, or both), and highlight that the alcohol oxygen becomes the ester oxygen while the acid OH leaves as water.
FAQ
Why is removing water so critical?
Fischer esterification is an equilibrium process. Any water present pushes the reaction backward (ester hydrolysis). Removing H₂O (Dean–Stark, sieves, azeotropic distillation) or using a massive excess of alcohol is the only way to favor ester formation.
Can I perform Fischer esterification with phenols or tertiary alcohols?
Phenols are weak nucleophiles and often fail under Fischer conditions; activate the acid (acid chloride, anhydride) or use DCC/DMAP instead. Tertiary alcohols tend to dehydrate or rearrange under acid/heat, so consider milder coupling agents.
Why does isotope tracing show that the alcohol oxygen ends up in the ester?
In the tetrahedral intermediate, the ROH oxygen stays bonded to carbon when water leaves. The acid OH oxygen departs as H₂O—classic ^18O-labeling experiments confirm this assignment.
Interactive Toolbox
- Mechanism Solver — Replay all seven RDKit steps, switch catalysts, and visualize water removal strategies.
- Reaction Solver — Compare excess-ROH vs Dean–Stark presets and predict whether the ester or hydrolysis dominates.
- IUPAC Namer — Confirm names such as methyl benzoate, ethyl cyclohexanecarboxylate, or γ-butyrolactone.
Related Reading
- Carboxylic Acid → Acid Chloride with SOCl₂ — activate the acid before coupling to sensitive alcohols.
- Carboxylic Acids → Primary Alcohols with LiAlH₄ — reduce acids completely when esterification is undesired.
- Acid Chlorides → Esters with ROH/Pyridine — convert activated acyl chlorides to esters under basic conditions.