Carboxylic Acids → Primary Alcohols with LiAlH₄
Lithium aluminum hydride (LiAlH₄, “LAH”) converts carboxylic acids (RCO₂H) into primary alcohols (RCH₂OH). The hydride reagent first acts as a base (acid–base step with H₂ evolution), then delivers two nucleophilic hydrides through an aluminum-carboxylate complex. Aqueous/acidic workup liberates the neutral alcohol. The aldehyde stage is never isolated; it exists only while coordinated to aluminum.
Quick Summary
| Reagents / conditions | LiAlH₄ (1.5–3.0 eq) in dry Et₂O/THF, 0 °C → reflux under inert gas; staged quench (H₂O → dilute H₃O⁺). |
|---|---|
| Outcome | RCO₂H → RCH₂OH with H₂ evolution during the reaction and aluminum salts removed during workup. |
| Mechanism | (1) Acid–base → (2) Hydride acyl substitution → (3) Collapse to the bound aldehyde → (4) Second hydride → (5) Proton transfer with H₃O⁺ → (6) Workup liberates the alcohol. |
| Why it works | Al(III) coordination activates the acyl carbon and keeps the nascent aldehyde bound long enough for the second hydride. |
| Stereochemistry | The acyl carbon simply goes from sp² to sp³; no rearrangements or new chiral centers. |
| Contrasts & pitfalls | NaBH₄ is too weak for acids; LiAlH₄ also reduces esters, amides, nitriles, and epoxides, so chemoselectivity and careful quench technique are essential. |
Mechanism — Six RDKit Frames
Each SVG comes directly from the RDKit Mechanism Solver, so the curved arrows and overlays match the interactive experience.
Mechanistic Checklist (Exam Focus)
- Show the acid–base kickoff: hydride grabs the acidic proton → H₂ bubbles off.
- Depict an Al-carboxylate before hydride delivery (coordination activates the acyl carbon).
- Two hydrides hit the same carbon: first gives an Al-bound aldehyde, the second yields an alkoxide.
- Emphasize that the aldehyde never escapes—LiAlH₄ immediately pushes it to the alcohol.
- Include a careful H₃O⁺ workup arrow; quenching incorrectly is a safety risk.
- Contrast vs NaBH₄ (inactive toward acids) and BH₃·THF (acid-selective, gentler).
Worked Examples
Benzoic acid + LiAlH₄ → benzyl alcohol
Reactant
Reagent
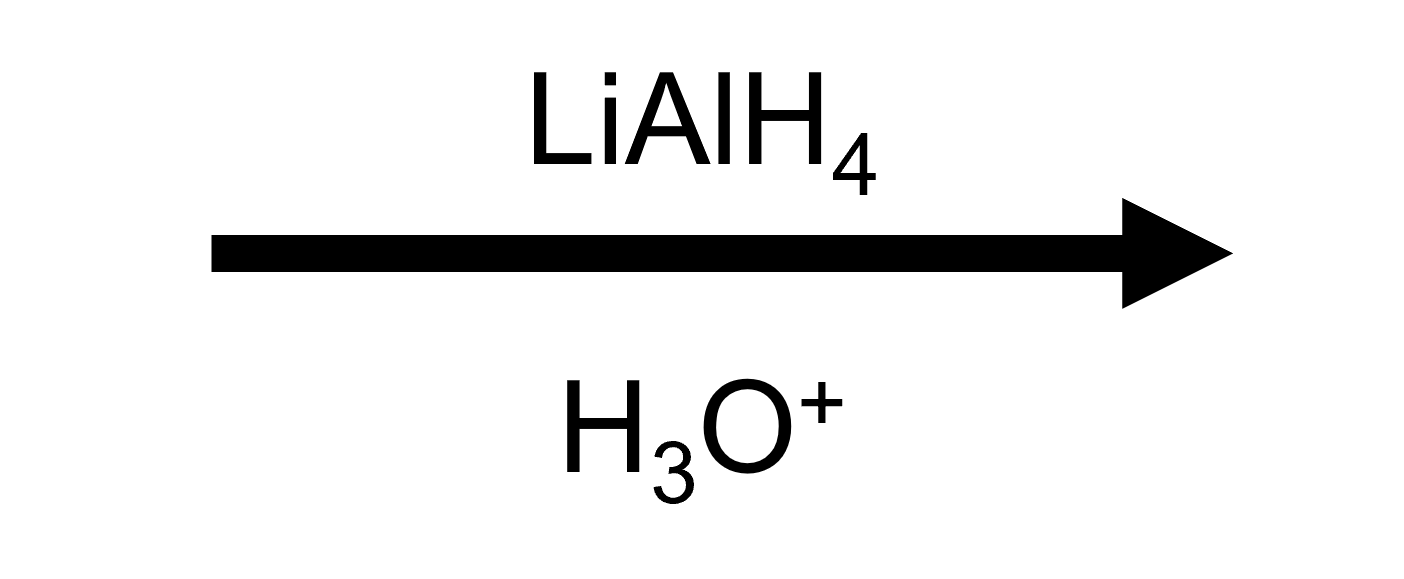
Product
Benzyl alcohol — the carboxylate carbon becomes the CH₂OH group.
Hexanoic acid → 1-hexanol
Reactant
Reagent
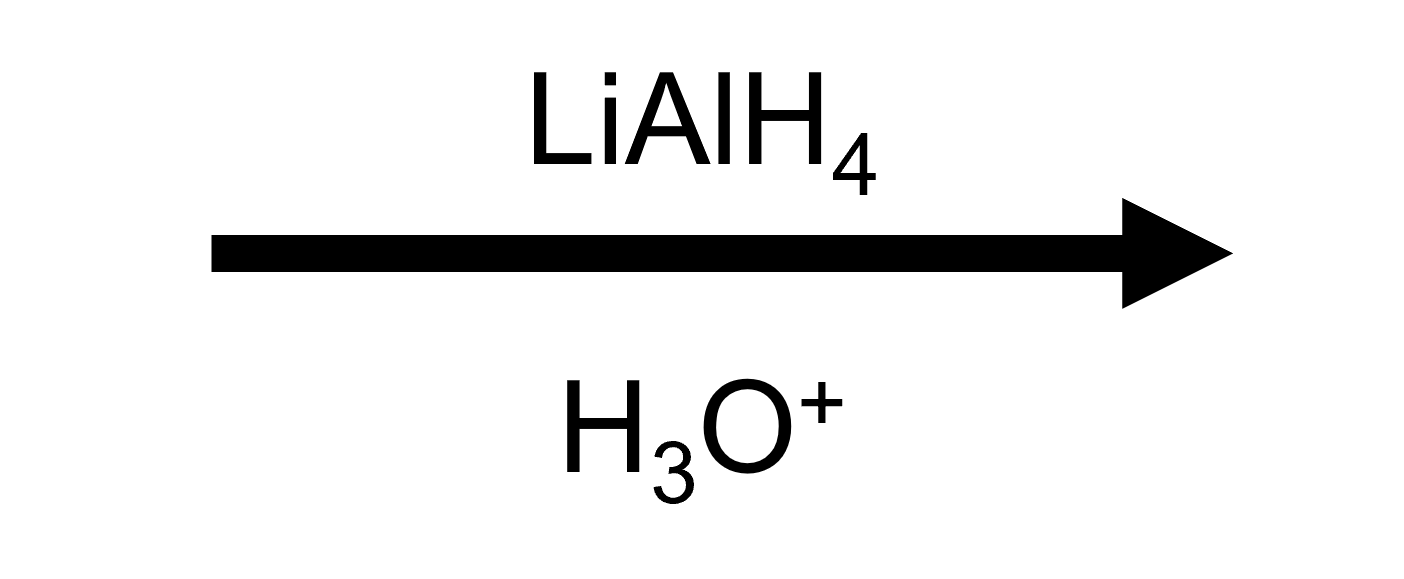
Product
2-Methylpropanoic acid → isobutanol
Reactant
Reagent
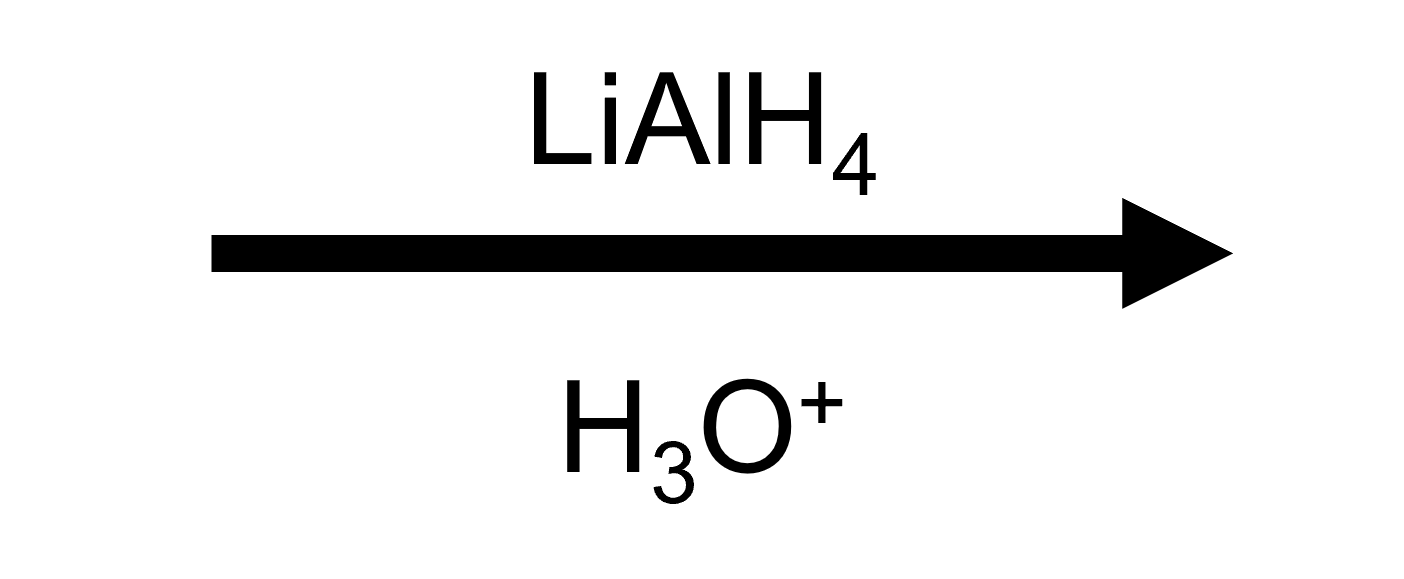
Product
Isobutanol — the reduction stops at the primary alcohol.
Scope & Limitations
- Broadly reduces: aliphatic/aromatic acids, diacids (→ diols), and polyfunctional acids (if no other reducible groups).
- Too reactive for selectivity: LiAlH₄ will also reduce esters, acid chlorides, amides, nitriles, epoxides, nitro groups, and some halides. Protect or sequence wisely.
- Aldehyde capture: cannot stop at an aldehyde; use LiAl(OR)₃H on acid chlorides or DIBAL-H on esters if you need RCHO.
- Protic handles: free –OH or –NH groups will quench the reagent—protect or deprotonate beforehand.
- Safety: reagent is pyrophoric when dry; every addition/quench must be ice cold and vented because H₂ evolves.
Practical Tips
- Dry everything. Oven-dry glassware, dry ether/THF, and an inert atmosphere before LiAlH₄ is exposed.
- Addition order: Slowly add the acid (or its solution) to a stirred suspension of LiAlH₄ at 0 °C. This keeps H₂ evolution manageable.
- Quench sequence: Classic Fieser-style quench—EtOAc (consumes excess hydride) → H₂O → dilute NaOH → H₃O⁺/NH₄Cl—prevents runaway exotherms.
- Solid workup: Expect aluminum salts; filter through Celite before concentrating.
- Plan hydride equivalents: One equivalent is lost to acid–base, so plan ≥2 equiv per carboxyl group (1.5–3.0 eq in practice).
Exam-Style Summary
Net reaction: RCO₂H —(LiAlH₄, dry ether/THF)→ RCH₂O⁻·Al —(H₃O⁺)→ RCH₂OH
Takeaways: hydride first acts as base (H₂), then delivers twice to the carbonyl. Aldehyde never leaves the coordination sphere, and a careful aqueous workup protonates the alkoxide. NaBH₄ is inert toward carboxylic acids.
FAQ
Can I isolate the aldehyde?
Not from a carboxylic acid with LiAlH₄—the aldehyde forms only as an aluminum complex and is immediately reduced. To stop at RCHO, transform the acid to an acid chloride and use LiAl(OR)₃H or apply DIBAL-H to an ester at −78 °C.
Why does NaBH₄ fail here?
Carboxylic acids are too weakly electrophilic and too acidic; NaBH₄ is rapidly quenched without delivering hydride. LiAlH₄ is both basic and a much stronger hydride donor.
What is the safe way to quench LiAlH₄ reductions?
Cool to 0 °C, add a proton source in stages (e.g., EtOAc → H₂O → dilute acid) behind a blast shield. Never dump water all at once—H₂ evolution plus exotherm can be violent.
Interactive Toolbox
- Mechanism Solver — Replay all six RDKit frames with overlays matching the article figures.
- Reaction Solver — Compare LiAlH₄ vs NaBH₄ vs LiAl(OR)₃H on carboxylic acids, esters, and acid chlorides.
- IUPAC Namer — Confirm the names for the resulting primary alcohols (benzyl alcohol, 1-hexanol, isobutanol, etc.).
Related Reading
- Carboxylic Acid → Acid Chloride with SOCl₂ — activate the acid before selective reductions or acylations.
- Acid Chloride → Aldehyde with LiAl(OR)₃H — swap to a bulky aluminum hydride when you need to stop at RCHO.
- Acid Chlorides → Esters with ROH/Pyridine — a common follow-up once the acid has been activated or reduced.