Epoxide Reactions: Base/Neutral Ring Opening (SN2-like)
Under basic or neutral nucleophilic conditions, epoxides open by an SN2-like pathway: a nucleophile attacks back-side at the less substituted epoxide carbon, the C–O bond to that carbon breaks, and the oxygen becomes an alkoxide. Protonation (from solvent or a separate workup) then furnishes the β‑substituted alcohol. The opening is anti (trans) relative to the departing C–O bond, so the attacked carbon inverts configuration. Strong nucleophiles—anionic heteroatoms, carbon-based anions, organometallics, or hydride donors—all follow this logic; protonating the epoxide first (acidic path) would reverse the regioselectivity.
Key Emphasis (Teaching Pivots)
- Regioselectivity (base/neutral): attack the less substituted epoxide carbon (classic SN2 preference); benzylic/allylic stabilization speeds the reaction but does not change the rule.
- Stereochemistry: back-side attack → inversion at the attacked centre → overall anti (trans) addition relative to the original epoxide.
- Workup awareness: ionic nucleophiles may protonate in situ (NaOMe/NaOEt use the matching ROH), whereas organometallics, acetylides, and LiAlH₄ require a dedicated acidic quench to reveal the neutral product.
- Counter-ion / reagent identity: swapping Na⁺ for K⁺ does not alter the regio rule, but it does change the spectator drawn in the mechanism, so we match each button to the reagent students recognize.
- No prior protonation: protonating the epoxide switches you into the acid-catalysed (Markovnikov) regime—keep strong acids away until workup.
Quick Summary
- Mechanism class: SN2-like epoxide opening of the unprotonated ring (closed-shell pathway).
- Regioselectivity: less substituted carbon in unsymmetrical epoxides; symmetry is the only exception.
- Stereochemistry: inversion at the attacked carbon; Nu and the newly formed OH end up trans.
- Outcome: β‑Nu alcohol after protonation/workup (Nu = CN, RS, RO/NaOMe/NaOEt, N₃, HO⁻/NaOH/KOH, hydride from LiAlH₄, organometallic carbon nucleophiles, etc.).
- Workup control: match the workup to the reagent (RO⁻ uses the conjugate ROH; LiAlH₄ demands H₃O⁺; simple NaOH/KOH quench in water).
- Contrast with acid: acid protonates the epoxide and directs attack to the more substituted carbon (SN1-like polarization); base does the opposite.
Mechanism — Base/Neutral Epoxide Opening (3 Frames; arrows A–F)
Mechanistic Checklist (Exam Focus)
- No protonation first: the ring stays neutral; Nu⁻ attacks directly.
- Less substituted carbon: default SN2 site unless the epoxide is symmetrical.
- Backside attack → inversion: mark inversion only at the attacked carbon; cyclic epoxides give trans products.
- Organometallics/hydride behave the same: RMgX, RLi, R₂CuLi, and LiAlH₄ attack the less substituted carbon; protonation occurs during workup.
- Solvent compatibility: polar aprotic solvents (DMF, DMSO, THF, MeCN) accelerate ionic nucleophiles; anhydrous ethers are mandatory for organometallics/LAH.
Worked Examples
2-Methyloxirane + NaOMe/MeOH → β-methoxy alcohol

MeO⁻ attacks the primary carbon anti; MeOH from the button protonates the alkoxide, revealing the β-methoxy alcohol.
2-Methyloxirane + NaOEt/EtOH → β-ethoxy alcohol
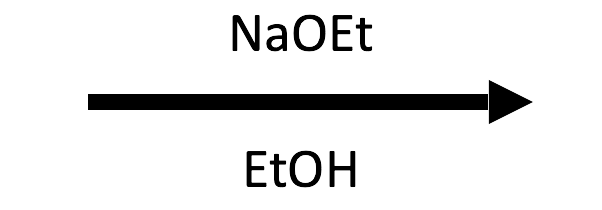
EtO⁻ opens at the less substituted carbon and EtOH delivers the proton, giving the anti β-ethoxy alcohol.
2-Methyloxirane + NaOH/H₂O → trans-1,2-diol
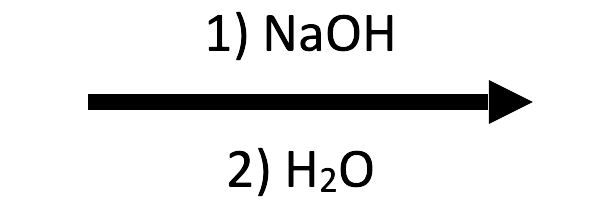
HO⁻ performs the SN2-like attack and water protonates the alkoxide, giving the anti diol featured in the NaOH/H₂O button.
2-Methyloxirane + KOH/H₂O → trans-1,2-diol (K⁺ overlay)
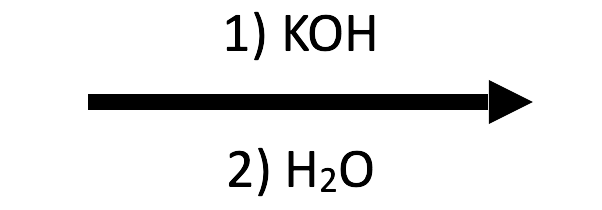
Switching to KOH changes only the spectator ion—the attack/workup sequence is identical, so the diol overlay now labels K⁺/OH⁻.
2-Methyloxirane + NaN₃/H₂O → β-azido alcohol
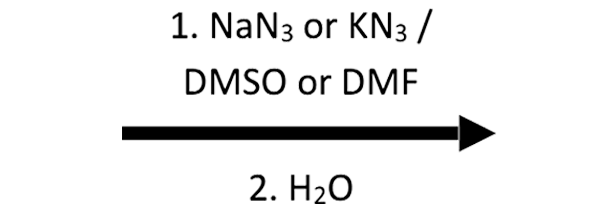
N₃⁻ approaches anti, giving the β-azido alcohol after water protonates the alkoxide; this sets up a later reduction to a β-amino alcohol.
2-Methyloxirane + LiAlH₄, then H₃O⁺ → reduced alcohol
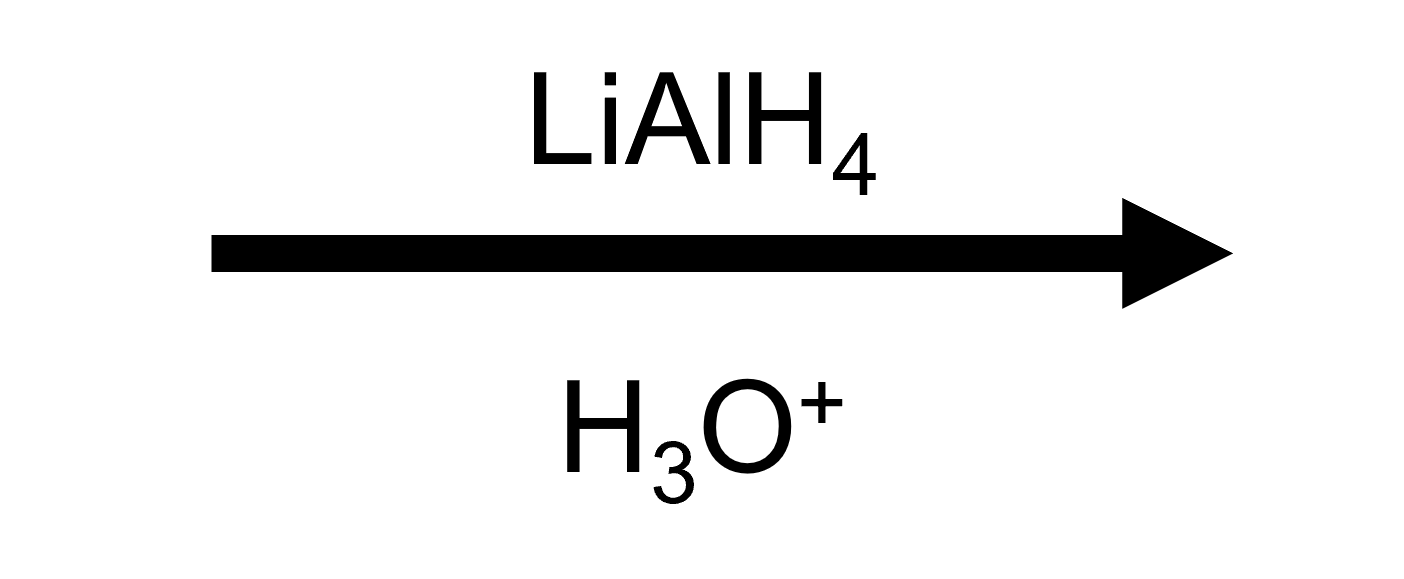
LiAlH₄ donates hydride to the less substituted carbon; only the H₃O⁺ workup (built into the button) reveals the neutral β-alkyl alcohol.
Scope & Limitations
- Works well: aryl, alkyl, and cyclic epoxides; polar aprotic solvents accelerate ionic nucleophiles (DMF, DMSO, THF, MeCN). Cyclic epoxides give trans-1,2 products.
- Slower / less favourable: highly hindered tertiary carbons (pure SN2 is disfavoured) and substrates bearing strongly acidic groups that would quench the nucleophile.
- Organometallic compatibility: RMgX, RLi, R₂CuLi, and LiAlH₄ demand anhydrous ether solvents; any protic medium destroys the reagent.
- Benzylic/allylic epoxides: still open at the terminal carbon under base; acid flips the regioselectivity to the benzylic/allylic site.
Practical Tips & Pitfalls
- Match solvent to nucleophile: NaCN/NaN₃/NaSR prefer DMF/DMSO (or aqueous workups); alkoxides are comfortable in ROH; RMgX/RLi/LAH need dry THF/Et₂O.
- Workup discipline: always include an acidic quench for organometallic or hydride openings; otherwise you will isolate an alkoxide salt.
- Safety: NaN₃ and CN⁻ are toxic; thiolates smell terrible; organolithium and Grignard reagents are pyrophoric—keep them under inert atmosphere.
- Don’t mix paradigms: any strong acid in the reaction mixture will protonate the epoxide and switch you into the acid-assisted (Markovnikov) module.
Exam-Style Summary
Base/neutral epoxide openings are SN2-like: Nu⁻ attacks back-side at the less substituted carbon, the attacked centre inverts, and the C–O bond opens anti to give an alkoxide. Protonation/workup reveals the β‑substituted alcohol. Organometallics and LiAlH₄ follow the same script (attack, then acidic workup). Acid-assisted openings do the opposite for regioselectivity.
Interactive Toolbox
- Mechanism Solver — Use Mechanism Solver to see each step of the base/neutral epoxide ring-opening mechanism along with explanations for every frame.
- Reaction Solver — Use Reaction Solver to quickly find the product of any epoxide reacted with these base/neutral reagents.
- IUPAC Namer — Use IUPAC Namer to learn the naming ins and outs of epoxides and the resulting β-substituted alcohols.