Ester to Aldehyde (DIBAL-H, -78 C)
Exam Answer
- Reagents
- 1) DIBAL-H (cold) 2) H3O+
- Major outcome
- Ester to aldehyde (controlled)
- Selectivity
- Stopping point depends on temperature/equivalents/workup
Common traps
- Warm conditions or excess reagent can over-reduce to alcohol
- Do not confuse with LiAlH4 (ester to alcohol)
- Workup timing matters in stepwise problems
Ester reduction outcomes by reagent
Jump to full playbook| Reagent | Major product | Notes |
|---|---|---|
| DIBAL-H (-78 C, about 1 eq) | aldehyde | controlled stop |
| LiAlH4 | primary alcohol | strong; no stop at aldehyde |
| NaBH4 | no ester reduction | typical exam assumption |
Diisobutylaluminum hydride (DIBAL-H, DIBAH, i-Bu₂AlH) converts esters into aldehydes when the reaction is run cold (typically −78 °C in toluene, hexanes, Et₂O, or THF), the hydride charge is limited to ~1 equiv, and the quench is kept cold. DIBAL-H acts as both a Lewis acid and hydride donor: Al coordinates the carbonyl oxygen, a single hydride forms a tetrahedral Al–alkoxide complex (“hemiacetalate”), and a cold protic quench fragments the complex to release the aldehyde while the alkoxy fragment becomes an alcohol. If the mixture warms or excess hydride remains, a second hydride reduces the newly formed aldehyde to the primary alcohol.
Key Emphasis (Teaching Pivots)
- Temperature + equivalents control selectivity. Esters (and lactones) stop at aldehydes/lactols with ~1.0–1.2 equiv DIBAL-H at −78 °C plus a cold quench. Warming toward 0 °C or using >1.5 equiv drives over-reduction to primary alcohols/diols.
- Mechanistic picture. Al–O coordination activates the ester; one hydride generates a tetrahedral Al–alkoxide complex that remains “frozen” at −78 °C. Cold MeOH followed by cold aqueous NH₄Cl collapses the complex to aldehyde + ROH while Al becomes insoluble salts.
- Scope cues. Lactones become lactols (cyclic hemiacetals) under these conditions; further steps are needed to open the ring. Acid chlorides can also stop at aldehydes with cold DIBAL-H, but those belong in the acyl chloride playbook.
Quick Summary
- Reagents/conditions: DIBAL-H (1.0–1.2 equiv), toluene/hexanes/Et₂O/THF, −78 °C; slow addition to the cold ester solution; quench at −78 °C with MeOH (or i-PrOH), then cold aqueous NH₄Cl or Rochelle’s salt.
- Outcome: R–C(=O)OR′ → R–CHO + R′OH on quench. Lactones give lactols (hemiacetals).
- Over-reduction risk: >1.5–2 equiv DIBAL-H, letting the mixture warm before quench, or quenching hot usually converts the intermediate aldehyde to the primary alcohol.
- Comparison: LiAlH₄ reduces esters directly to alcohols; NaBH₄ is generally too mild. DIBAL-H sits between them—strong hydride, but single equivalent can be “frozen” at the aldehyde stage.
Mechanism — Partial Reduction and Quench Release







Mechanistic Checklist (Exam Focus)
- Show Al–O coordination followed by a single Al–H hydride arrow to the carbonyl carbon.
- Name/depict the tetrahedral Al–alkoxide intermediate—students should recognize it as the “frozen” species at −78 °C.
- Quench order matters: alcohol (MeOH/i-PrOH) at −78 °C, then cold aqueous NH₄Cl/dilute acid. Draw both steps or annotate them clearly.
- Track the leaving-group fragment: OR′ becomes R′OH on workup; in lactones the OR′ is part of the same ring, so the product is a lactol.
- Include the over-reduction trap: warm temperatures or excess hydride reduce the aldehyde further to the primary alcohol/diol.
Worked Examples

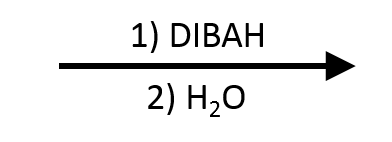


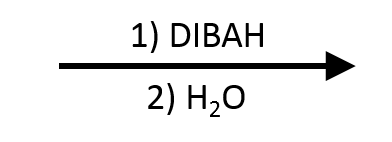


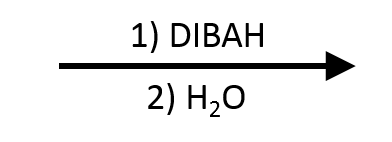

Scope & Limitations
- Works best: Aliphatic, benzylic, and aryl esters; benzyl/t-Bu esters stop particularly cleanly at aldehydes. Lactones give lactols that can later be opened/oxidized.
- Functional groups tolerated (pre-quench): Al–H reagents demand strictly aprotic, oxygen-free setups. Protect alcohols/phenols if they would react with DIBAL-H; nitriles can be reduced to imines (which hydrolyze to aldehydes) but that is a different substrate class.
- Temperature: Keep ≤−60 °C until after the first protic quench to avoid runaway hydride transfer.
- Over-reduction: >1.5 equiv hydride, warming before quench, or slow quench almost always give primary alcohols (or diols from lactones).
Edge Cases & Exam Traps
- Warm quench: Letting the mixture warm toward 0 °C before quenching effectively mimics LiAlH₄—expect primary alcohols.
- Excess reagent: Stock DIBAL-H solutions are often >1.0 M; miscalculations lead to >1.5 equiv and over-reduction.
- Lactone misassignment: At −78 °C you get a lactol, not an open-chain aldehyde, unless you subsequently open the ring or heat.
- Protic solvent before quench: EtOH, MeOH, or halogenated solvents prior to the planned quench destroy Al–H and derail the reaction.
Practical Tips
- Calibrate the reagent. Verify the actual concentration of the DIBAL-H solution and charge only 1.0–1.2 equiv for aldehyde stops.
- Add to cold substrate. Cool the ester solution to −78 °C first, then add DIBAL-H slowly while monitoring (TLC/GC when possible).
- Two-stage, cold quench: At −78 °C add a few equiv MeOH (or i-PrOH) to destroy excess hydride, then add cold aqueous NH₄Cl (or Rochelle’s salt/dilute acid). Warm only after Al salts appear.
- Workup: Filter off Al salts (Celite) before concentration to minimize redox side reactions.
- Safety: DIBAL-H is pyrophoric; work behind a blast shield, maintain positive N₂ flow, and vent carefully during quench.
Exam-Style Summary
Ester + DIBAL-H (≈1.0 equiv, −78 °C) → tetrahedral Al–alkoxide (frozen) → cold MeOH then cold NH₄Cl → aldehyde + ROH. Excess hydride or warming delivers the primary alcohol/diol. Lactones give lactols under the cold protocol.
Interactive Toolbox
- Mechanism Solver — Toggle DIBAL-H equivalents (1.0 equiv vs 2.0 equiv) and temperature (−78 °C vs 0 °C) to see the aldehyde vs alcohol pathway, plus an optional lactone mode for lactols.
- Reaction Solver — Provide an ester (or lactone) and the Solver predicts aldehyde vs alcohol outcomes based on your temperature/equivalent inputs.
- IUPAC Namer — Use it to caption the aldehyde and alcohol products (no SMILES shown to end-learners).