Ester to Primary Alcohol (LiAlH4)
Exam Answer
- Reagents
- 1) LiAlH4 2) H3O+
- Major outcome
- Ester to primary alcohol (plus alcohol from leaving group)
- Selectivity
- Strong reducer; does not stop at aldehyde
Common traps
- No alcohol product without aqueous workup
- LiAlH4 does not stop at aldehyde (use DIBAL-H for that)
- Other reducible groups may also be reduced
Ester reduction outcomes by reagent
| Reagent | Major product | Notes |
|---|---|---|
| LiAlH4 | primary alcohol | strong; goes past aldehyde |
| DIBAL-H (cold, about 1 eq) | aldehyde | control temperature and equivalents |
| NaBH4 | no ester reduction | typical exam assumption |
Lithium aluminum hydride (LiAlH₄, LAH) is a powerful hydride donor that transforms esters into primary alcohols. The sequence is polar and concerted: one hydride adds to the ester carbonyl to form a tetrahedral Al–alkoxide, the alkoxide collapses to an aldehyde (expelling the –OR′ fragment as an aluminum alkoxide), and a second hydride reduces that aldehyde to a primary alkoxide. Only after the reduction is complete do we add water/acid to protonate the alkoxides and reveal the free alcohols. Because aldehydes react faster than esters with LAH, the aldehyde intermediate cannot be isolated—it is immediately consumed by the second hydride addition.
Key Emphasis (Teaching Pivots)
- Two hydrides per ester carbonyl. The first hydride forms a tetrahedral intermediate that collapses to an aldehyde; the second hydride reduces that aldehyde to an alkoxide. Aldehyde isolation is impossible under LAH conditions.
- Two alcohols after work-up. The acyl fragment becomes the primary alcohol, and the –OR′ leaving group is protonated to R′OH during the quench.
- Operational reality. Absolutely anhydrous ether/THF, inert atmosphere, and staged quench (MeOH or EtOAc → H₂O → dilute acid). Hydronium is never present during the reduction itself.
- Contrast reagents. NaBH₄ is generally too mild for esters; cold DIBAL-H can stop at an aldehyde (handled in the companion article).
Quick Summary
- Reagents/conditions: 1) LiAlH₄ (≥2 hydrides per ester), dry Et₂O or THF, 0 °C → rt. 2) Careful quench (often i-PrOH or EtOAc first) followed by H₂O and H₃O⁺/NH₄Cl.
- Outcome: R–COOR′ → R–CH₂OH + R′OH after protonation; lactones produce diols.
- Stoichiometry: Minimum two hydrides per carbonyl; in practice LAH is used in excess to offset side consumption and quench.
- Safety: Add LAH last, exclude protic solvents until the quench, and vent for H₂ evolution during work-up.
Mechanism — Five Steps (LiAlH₄ Sequence)





Step commentary:
- Step 1 (A–B). Al–H donates hydride to the carbonyl carbon while the π bond shifts to oxygen. Aluminum remains coordinated to the oxygen, stabilizing the alkoxide.
- Step 2 (C–D). The alkoxide lone pair collapses, ejecting OR′ as an Al-bound alkoxide and generating the aldehyde in situ.
- Step 3 (E). A fresh Al–H (often from the same LAH cluster) reduces the aldehyde to a primary alkoxide (R–CH₂O⁻·Al).
- Step 4 (F–G). Only after complete reduction do we add protic reagents. A controlled quench (alcohol → H₂O → acid) protonates each alkoxide while destroying residual LAH.
- Step 5. Protonated products appear: the acyl fragment is now R–CH₂OH, and the leaving group becomes R′OH (or both positions become hydroxyls in the case of lactones).
Mechanistic Checklist (Exam Focus)
- Count two hydrides per ester carbonyl; note the aldehyde is formed but immediately reduced.
- Two alcohols result after quench: R–CH₂OH (acyl fragment) and R′OH (leaving group).
- Never show H₃O⁺ during the reduction—only during work-up.
- LAH reductions are closed-shell; no radicals/cations, so draw curved arrows accordingly.
- Lactones furnish diols (ring opening) under the same sequence.
Worked Examples
Example A — Methyl benzoate → benzyl alcohol + methanol
Classic aromatic ester: two hydrides reduce the benzoyl fragment to benzyl alcohol; methanol comes from the methoxy leaving group.

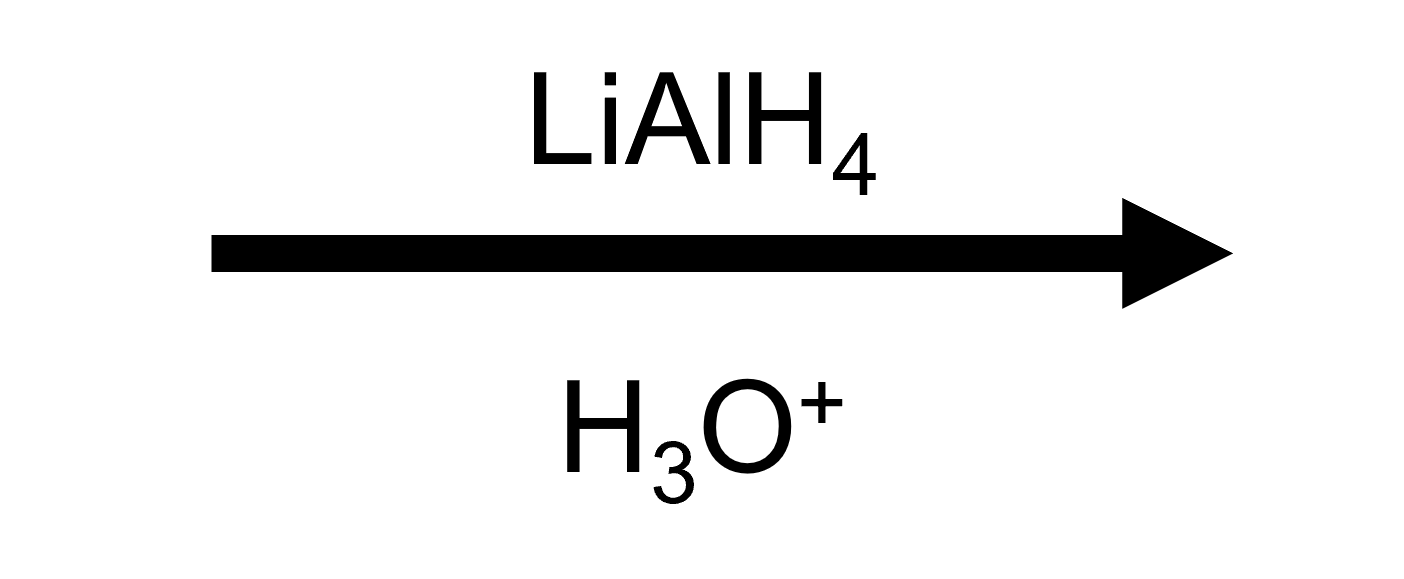

Example B — Ethyl acetate → two equivalents of ethanol
The acyl portion becomes ethanol (via CH₃CH₂–C=O), and the ethoxy leaving group protonates to ethanol as well. The work-up reveals two identical alcohols.

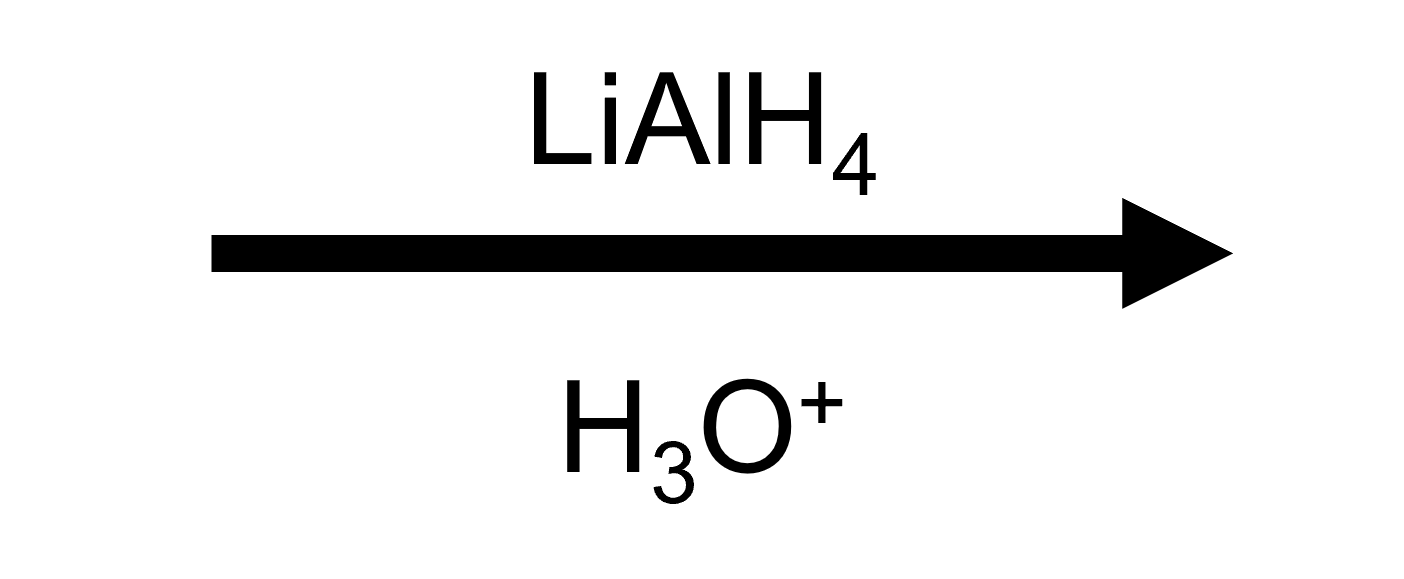

Example C — δ-Valerolactone (oxan-2-one) → 1,5-pentanediol
Lactones behave like intramolecular esters: LiAlH₄ opens the ring, adding two hydrides to give a longer diol under the standard quench.

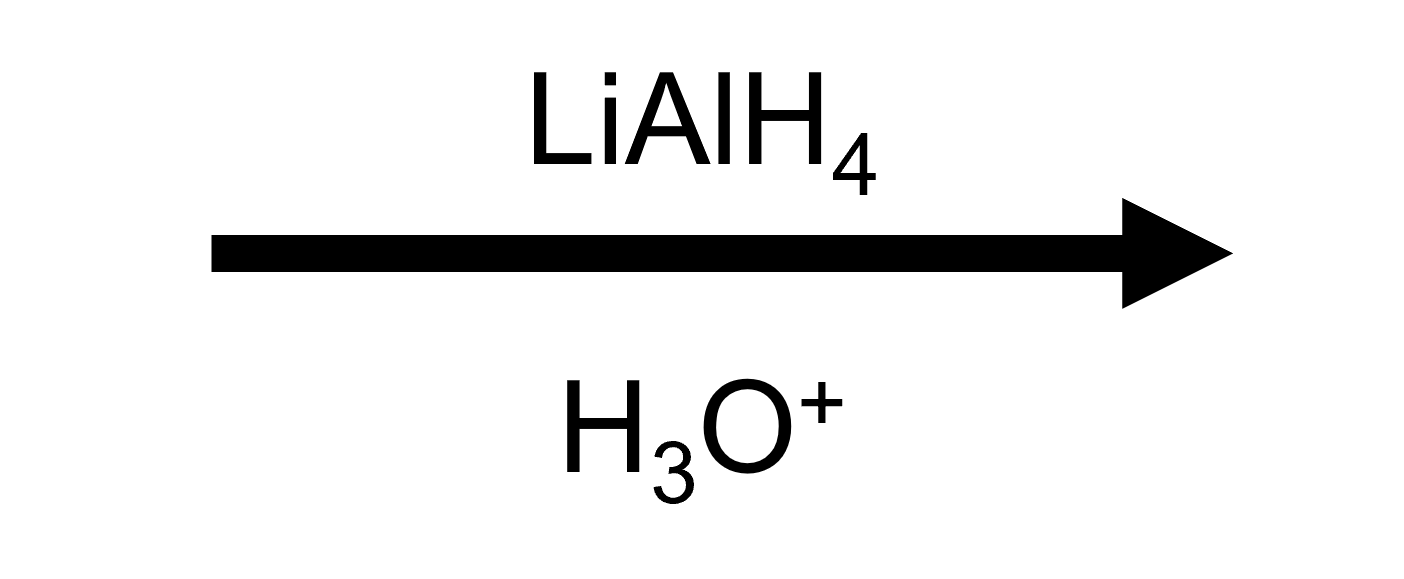

Scope & Limitations
- Reliable: Simple alkyl/aryl esters, lactones, diesters (each carbonyl consumes two hydrides), acid chlorides, and anhydrides all reduce rapidly.
- Also reduced by LAH: Carboxylic acids → primary alcohols; amides and nitriles → amines; epoxides → alcohols. Protect or sequence functional groups accordingly.
- Chemoselectivity: Aldehydes and ketones react faster—if they are present, they will be reduced before the ester.
- Conjugation: α,β-Unsaturated esters undergo 1,2-reduction of the carbonyl; extended forcing conditions may also reduce the C=C.
- Not suitable when you need an aldehyde intermediate. Use cold DIBAL-H instead for ester → aldehyde stops.
Edge Cases & Exam Traps
- Drawing H₃O⁺ or H₂O in the reaction mixture (instead of during the quench).
- Claiming the aldehyde can be isolated under LAH—only external reagents (e.g., DIBAL-H) can stop there.
- Forgetting the leaving group alcohol in the products list.
- Proposing NaBH₄ as an alternative for esters (it is too mild under standard undergraduate conditions).
Practical Tips
- Dry, inert setup (oven- or flame-dried glassware, N₂/Ar blanket) and add LAH last to a cooled solution (0 °C) of the ester in THF or Et₂O.
- Charge enough hydride (≥2 equiv per ester carbonyl) to allow for reaction + controlled quench.
- Quench sequence: often EtOAc or i-PrOH dropwise at 0 °C (destroys excess hydride) → ice/H₂O → dilute acid/NH₄Cl. Expect vigorous H₂ evolution.
- Filter aluminum salts (Celite) prior to concentration; Rochelle’s salt can help break emulsions.
- Store LAH properly—it's pyrophoric and reacts violently with moisture.
Exam-Style Summary
LiAlH₄ reduces esters to primary alcohols via two hydride transfers and a fleeting aldehyde intermediate. The acyl fragment becomes R–CH₂OH, the –OR′ group becomes R′OH, and a staged aqueous/acidic work-up reveals both products. Remember: no hydronium is present during the reduction itself—only during the quench.
Interactive Toolbox
- Mechanism Solver — Load the “Ester → Alcohol (LiAlH₄)” flow to watch each hydride delivery, aldehyde collapse, second reduction, and the staged quench. Use the controls to emphasize the transient aldehyde.
- Reaction Solver — Compare LiAlH₄ with DIBAL-H or NaBH₄ on the same ester to see which conditions stop at aldehydes versus full reduction to R–CH₂OH + R′OH.
- IUPAC Namer — Generate systematic names for both alcohol products (acyl fragment + leaving-group alcohol) to use in written explanations or flashcards.