Ester Reactions: Acid Hydrolysis (Ester → Carboxylic Acid)
Acid-catalyzed hydrolysis is the reverse of Fischer esterification. Protonating the carbonyl oxygen increases electrophilicity; water adds to give a tetrahedral oxonium; rapid proton shuttles convert that intermediate into the form where ROH is protonated and primed to depart; collapse re-forms the carbonyl and expels ROH; deprotonation reveals the carboxylic acid and regenerates H₃O⁺. Because the process is reversible, excess water and/or removal of ROH (distillation, extraction) drive the equilibrium towards hydrolysis.
Key Emphasis (Teaching Pivots)
- Reversibility / equilibrium. This is Fischer hydrolysis. Product ratios are equilibrium-controlled; use excess H₂O or remove ROH to favor RCO₂H formation.
- Mechanistic spine. Protonate C=O → H₂O addition → proton-shuffled tetrahedral intermediates → collapse expelling ROH → deprotonate to regenerate H₃O⁺.
- Rate logic. Acid makes the acyl carbon more electrophilic and turns OR′ into ROH (a better leaving group) via protonation.
- Exception worth memorizing. tert-Alkyl esters can hydrolyze via alkyl–oxygen cleavage (AAL1/SN1) with R₃C⁺ formation, especially t-Bu esters used as acid-labile protecting groups.
Quick Summary
- Reagents/conditions: H₃O⁺ (aq) catalytic; 0 °C → reflux depending on substrate; run with excess water or remove ROH to drive hydrolysis.
- Outcome: R–C(=O)OR′ + H₂O ⇌ R–C(=O)OH + R′OH (acid catalyst regenerated).
- Special case: t-Bu esters (and other tertiary/benzylic esters) may hydrolyze by AAL1/SN1 alkyl–oxygen cleavage, often very fast under strong acid.
Mechanism — Six Steps (Acid Addition–Elimination)






Step commentary:
- Step 1. Oxonium formation (protonate C=O) is the “activation” step for both directions of Fischer chemistry.
- Step 2. Water (not HO⁻) attacks the protonated carbonyl, producing a tetrahedral oxonium.
- Step 3. Proton transfers interconvert the TIs so that ROH is the weaker base and therefore the leaving group.
- Step 4. Lone pair on the acyl oxygen begins the collapse while ROH remains protonated.
- Step 5. Collapse completes: ROH leaves and the protonated acid remains.
- Step 6. Deprotonation of the acyl group gives RCO₂H and regenerates the acid catalyst.
Mechanistic Checklist (Exam Focus)
- Reversible with Fischer esterification; cite Le Châtelier control (excess H₂O or removal of ROH).
- Addition–elimination at the acyl carbon: always pass through the tetrahedral intermediate.
- Leaving group logic: protonated ROH leaves more easily than H₂O in acid.
- Acid’s role: increase electrophilicity and protonate OR′; no HO⁻ in the mechanism.
- Exception: t-Bu (and other tertiary/benzylic) esters often hydrolyze via AAL1/SN1 at the alkyl–O bond.
Worked Examples
Ethyl acetate → acetic acid + ethanol
Classic Fischer hydrolysis: reflux in aqueous H₃O⁺, use water as solvent or distill ethanol to pull the equilibrium.

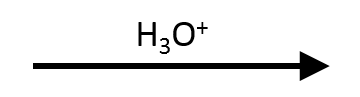

Methyl benzoate → benzoic acid + methanol
Aryl esters hydrolyze readily; warm aqueous acid and excess water are sufficient.

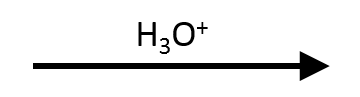

t-Butyl p-methoxybenzoate → p-anisic acid + t-BuOH (AAL1)
Strong acid, minimal water: protonate the alkoxy oxygen, form t-Bu⁺ (AAL1/SN1), capture with H₂O to give t-BuOH / isobutene while the acyl fragment becomes the free acid.

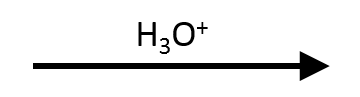

Scope & Limitations
- Works well: Aliphatic and aryl esters; lactones give hydroxy acids (ring opening). Transesterifications follow the same manifold but use ROH as the nucleophile.
- Fast substrates: Aryl esters (better acyl activation) and tert-alkyl esters (AAL1) hydrolyze rapidly.
- Sensitive groups: Acid-labile protecting groups (acetals, t-Boc) may fall off—plan the order of operations.
- Equilibrium caveat: In ROH solvent the reaction can run backwards or transesterify.
Edge Cases & Exam Traps
- Drawing RO⁻ as a leaving group in acid (should be ROH or ROH₂⁺).
- Forgetting that hydrolysis is reversible; do not promise “quantitative” conversion without Le Châtelier reasoning.
- Missing the tert-alkyl/benzylic (AAL1/SN1) cleavage path.
- Confusing acidic hydrolysis with basic saponification (irreversible, yields carboxylate).
Practical Tips
- Use excess water and heat; co-distill or extract ROH to drive hydrolysis.
- Keep acid catalytic (typically 5–50 mol % strong mineral acid). Too much acid promotes dehydration or deprotection elsewhere.
- For t-Bu esters, p-TsOH or TFA under anhydrous conditions cleanly removes the protecting group via AAL1.
- After hydrolysis, neutralize and extract; adjust pH to isolate the free acid.
Exam-Style Summary
H₃O⁺ protonates the ester carbonyl → H₂O adds → proton transfers set up ROH as the leaving group → collapse re-forms C=O and expels ROH → deprotonation yields RCO₂H. The reaction is reversible with Fischer esterification; push toward acids with excess water or ROH removal. Remember the tert-alkyl AAL1 exception.
Interactive Toolbox
- Mechanism Solver — Animate Steps 1–5 for acid hydrolysis and toggle the “tert-alkyl (AAL1)” branch to see alkyl–oxygen cleavage.
- Reaction Solver — Compare acid hydrolysis with base saponification or esterification by adjusting H₂O/ROH equivalents and temperature.
- IUPAC Namer — Confirm the systematic names of both the carboxylic acid and the liberated alcohol for reports or flashcards.