Carboxylate to Carboxylic Acid (Acid Workup)
Exam Answer
- Reagents
- H3O+ (acid workup)
- Major outcome
- RCO2- to RCO2H
- Selectivity
- Workup changes protonation state, not carbon skeleton
Common traps
- Not oxidation or reduction: just protonation
- Product may be drawn as a salt if not acidified
- Extraction depends on protonated vs deprotonated form
Treating an ester with hot aqueous NaOH performs saponification, a base-promoted nucleophilic acyl substitution (BAc2). Hydroxide attacks the carbonyl to give a tetrahedral intermediate that collapses, expelling RO⁻ and generating a carboxylic acid that is immediately deprotonated to its carboxylate salt. Because the product is RCO₂⁻ (a very poor leaving group), the reaction is effectively irreversible. After the base stage is complete, a separate acidic work-up (H₃O⁺) protonates the carboxylate to furnish the neutral carboxylic acid.
Key Emphasis (Teaching Pivots)
- Mechanistic class: Base-promoted nucleophilic acyl substitution (BAc2): HO⁻ adds → tetrahedral intermediate → collapse expels RO⁻ → carboxylate forms.
- Irreversibility: Deprotonation to RCO₂⁻ removes the electrophile and makes the reverse process (re-esterification) inaccessible under base; one equivalent of base is consumed.
- Workflow: Stage 1 (NaOH, heat) delivers the carboxylate salt + ROH. Stage 2 (H₃O⁺ work-up) protonates the salt to the free carboxylic acid.
- Contrast to acid hydrolysis: Acidic hydrolysis is equilibrium-controlled and reversible; saponification is effectively one-way.
Quick Summary
- Reagents/conditions: (1) NaOH (aq), reflux/heat; (2) H₃O⁺ acidic work-up.
- Stage 1 outcome: R–C(=O)OR′ + OH⁻ → R–CO₂⁻ + R′OH (Na⁺ balances the carboxylate). Base is consumed stoichiometrically.
- Stage 2 outcome: R–CO₂⁻ + H₃O⁺ → R–CO₂H + H₂O. The alcohol by-product remains the same.
- Driving force: Formation of the carboxylate salt (poor leaving group) locks the reaction products.
Mechanism — Four Frames (Base Stage + Acidic Work-Up)




Mechanistic Checklist (Exam Focus)
- Base is not catalytic: each ester carbonyl consumes one equivalent of HO⁻ (which becomes ROH).
- Leave RO⁻/ROH explicitly — the leaving group departs as an alkoxide, then becomes the alcohol coproduct.
- Emphasize the carboxylate after Stage 1 (no neutral acid until work-up).
- Do not draw protonated carbonyls or carbocations under base; everything is closed-shell.
- Irreversibility arises because RCO₂⁻ will protonate any alkoxide faster than the reverse addition–elimination can occur.
Worked Examples
Methyl benzoate → sodium benzoate + methanol (then benzoic acid)
Hot NaOH converts methyl benzoate into sodium benzoate (the carboxylate salt) and methanol; H₃O⁺ work-up furnishes benzoic acid.

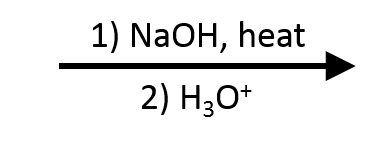

Ethyl acetate → sodium acetate + ethanol (then acetic acid)
Saponification of ethyl acetate is textbook: the acetate salt forms under base, and acidification liberates acetic acid while ethanol remains the coproduct.

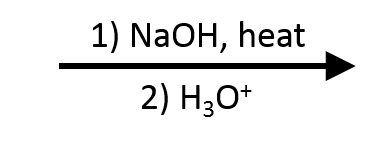

γ-Butyrolactone → sodium 4-hydroxybutanoate (then 4-hydroxybutanoic acid)
Lactones behave like intramolecular esters: NaOH opens the ring to the hydroxy-carboxylate; acidification delivers 4-hydroxybutanoic acid.

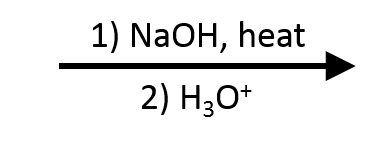

Scope & Limitations
- Works well: Simple alkyl/aryl esters, triglycerides (fat/oils → soaps), and lactones (ring-open to hydroxy acids).
- Slower substrates: Strongly deactivated or very hindered acyl groups may require longer reflux or higher base concentration.
- Selectivity: Other base-labile functional groups (acid chlorides, anhydrides) will react even faster. Amides are significantly less reactive and typically survive.
- Solvent effects: Using RO⁻/ROH instead of HO⁻/H₂O leads to transesterification rather than hydrolysis.
Edge Cases & Exam Traps
- Reporting the neutral acid before work-up (incorrect); the base stage ends at the carboxylate salt.
- Drawing SN2 at the alkyl carbon (wrong pathway) instead of addition–elimination at the carbonyl carbon.
- Forgetting that one equivalent of base is consumed (overall stoichiometry).
- Confusing the irreversible base route with the reversible acid-catalyzed hydrolysis.
Practical Tips
- Use aqueous NaOH or KOH at reflux (H₂O or H₂O/EtOH). Provide good mixing when the ester is hydrophobic (phase transfer or vigorous stirring).
- After saponification is complete, cool and acidify to pH < 2 with H₃O⁺ (often dilute HCl). Many carboxylic acids precipitate and can be filtered.
- For soap making, isolate the sodium (or potassium) carboxylate salts directly; acidifying later regenerates the free fatty acids.
- Avoid depicting H₃O⁺ during the base stage; introduce it only during the work-up panel.
Exam-Style Summary
(1) NaOH, heat: HO⁻ adds to the ester, collapse expels RO⁻, and the carboxylic acid is trapped as RCO₂⁻ (plus R′OH). (2) H₃O⁺ work-up protonates the carboxylate to give the isolated carboxylic acid. Irreversible because carboxylate is a terrible leaving group under basic conditions.
Interactive Toolbox
- Mechanism Solver — Use Mechanism Solver to animate hydroxide attack, collapse, carboxylate formation, and the H₃O⁺ work-up (toggle lactone mode for ring opening).
- Reaction Solver — Use Reaction Solver to compare irreversible base hydrolysis with equilibrium-driven acid hydrolysis on the same substrate.
- IUPAC Namer — Use IUPAC Namer to practice naming the carboxylate salts (Stage 1) and the neutral acids after work-up.