Grignard Reagents + Esters → Tertiary (or Secondary) Alcohols
Grignard reagents (RMgX) behave as carbon nucleophiles and strong bases. When they meet an ester in rigorously anhydrous ether or THF, two sequential nucleophilic additions take place: the first forms a tetrahedral intermediate that collapses to a ketone, and the second converts that ketone into a tertiary alkoxide. Only after aqueous H₃O⁺ workup does the neutral alcohol appear. Every textbook example hinges on this “over-add” logic, and the only common exception is the formate ester (HCOOR″), which yields a secondary alcohol because one substituent is hydrogen.
Key Emphasis (Learning Pivots)
- Two equivalents minimum. The second equivalent is consumed by the ketone intermediate; without it, the ketone simply waits to be attacked by any remaining RMgX.
- Formate exception. A formate ester (HCOOR″) delivers a secondary alcohol because the carbinol carbon keeps one hydrogen.
- No “ketone stop.” Ketones formed in Step 2 are more electrophilic than the starting ester, so “using one equivalent” rarely halts the sequence—use Weinreb amides for that.
- Strictly anhydrous. Acidic or protic functionality (–OH, –NH, –CO₂H, terminal alkyne) instantly destroys RMgX; plan the synthesis order or protect those groups.
Quick Summary
- Reagents & conditions: ≥2.0 equiv RMgX, dry Et₂O or THF, 0 °C → rt under inert gas; then cold H₃O⁺ (or sat. NH₄Cl) workup.
- Outcome:
- General esters → tertiary alcohol in which the OH carbon bears two identical groups from RMgX and one from the acyl fragment.
- Formate esters → secondary alcohol (R–CH(OH)–R) because the formyl hydrogen persists.
- Mechanistic arc: Addition → collapse (ketone) → second addition → tertiary alkoxide → protonation.
- Stereochemistry: Addition into a planar C=O produces racemic mixtures unless a chiral auxiliary or reagent is used.
- Common pitfalls: Trying to isolate the ketone, ignoring acidic impurity quenching, or forgetting to scale RMgX for diesters/lactones.
Mechanism — Five Steps of Over-Addition
Mechanistic Checklist
- Show two discrete additions in mechanisms; the expelled alkoxide should be depicted as R″O−·MgX⁺ (or its protonated ROH form during workup).
- If diesters or lactones are present, each carbonyl requires ≥2 equiv RMgX.
- Keep closed-shell arrows; there are no radicals—RMgX addition is a polar process.
- The workup (NH₄Cl(aq), dilute H₃O⁺, or H₂O) must be drawn; otherwise the on-paper species is an alkoxide, not an alcohol.
- Emphasize that ketones are transient; to stop at a ketone, switch to a Weinreb amide or acid chloride + cuprate strategy.
Worked Examples (IUPAC Descriptions)
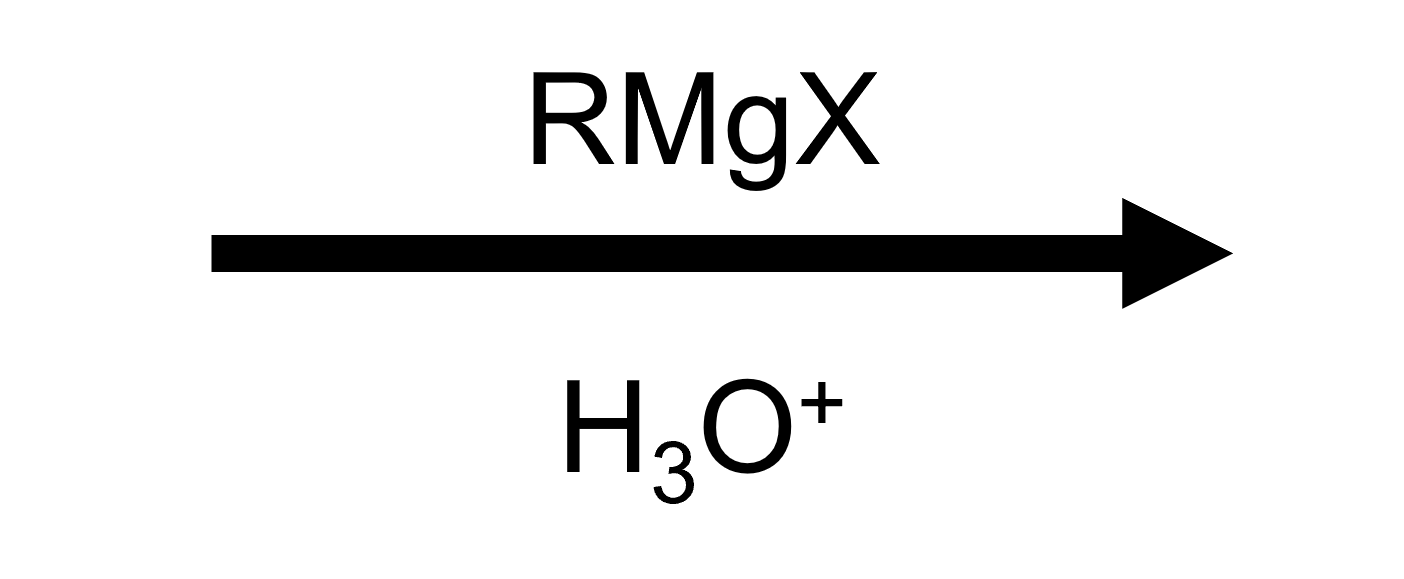
Worked sequences reinforce the synth-logic: count the number of carbonyls, the number of equivalents, and whether the ester is a formate.
Scope & Selectivity Notes
- Substrate classes: Alkyl or aryl esters, formates, and lactones all participate. Lactones open to give diols after two additions plus workup.
- Functional-group compatibility: Protect or sequence around acidic protons. Carbonyls more reactive than esters (acid chlorides, anhydrides) will also be consumed—plan accordingly.
- Polyesters/diesters: Scale RMgX stoichiometry (≥4 equiv for diesters) or handle each carbonyl sequentially.
- Chemoselectivity tip: To install one R group, convert the ester into a Weinreb amide or perform a cuprate acylation; Grignards will over-add.
Practical Tips & Pitfalls
- Dryness is non‑negotiable. Flame-dry glassware, use freshly distilled Et₂O/THF, and maintain inert gas.
- Addition order: Adding ester to RMgX moderates exotherms and keeps RMgX in slight excess when the ketone forms.
- Temperature control: Start at 0 °C, then warm to rt after addition. Excess heat accelerates side reactions and decomposition.
- Quench strategy: Cool the mixture, quench with NH₄Cl(aq) or dilute H₃O⁺, then follow with aqueous workup to remove Mg salts.
- Stoichiometry reminders: Reserve extra RMgX for diesters, lactones, or when other electrophiles lurk in the substrate.
Exam-Style Summary
Ester + ≥2 equiv RMgX, ether solvent, then H₃O⁺ → tertiary alcohol (secondary from formates). Mechanism: addition → collapse (ketone) → addition → protonation. Ketone cannot be isolated; show racemic products when new stereocenters form; cite Weinreb amides when the problem explicitly asks for “stop at ketone.”
Interactive Toolbox
- Mechanism Solver — Load the
rmgx_h3o.pngreagent button to watch each step of the RMgX + ester sequence with the new spacing and protonation arrows. - Reaction Solver — Forecast products for ester vs Weinreb-amide substrates under RMgX conditions and surface incompatibilities before the lab run.
- IUPAC Namer — Verify product names such as triphenylmethanol or tert-butan-2-ol drawn in the worked examples.
FAQ & Exam Notes
- Why does the reaction over-add? The intermediate ketone is more electrophilic and less hindered at oxygen than the starting ester, so any leftover RMgX immediately attacks it.
- Can I stop at the ketone with an ester? Practically no—switch to a Weinreb amide or an acid chloride + cuprate combination to obtain a single addition.
- Which alcohol class results? Most esters give tertiary alcohols (two identical R groups from RMgX). Formate esters uniquely give secondary alcohols.
- What destroys RMgX? Any protic functionality (–OH, –NH, –CO₂H) or even moisture; they protonate the carbanion instantly.
- How do lactones behave? The same two additions occur, but ring opening leads to diols after workup—plan stoichiometry accordingly.