EWG vs EDG: Examples of Electron Withdrawing and Donating Groups
Electron Withdrawing Groups vs. Electron Donating Groups: Key Differences and Effects in Organic Chemistry
Understanding electron withdrawing groups (EWGs) and electron donating groups (EDGs) is essential for mastering reactivity, resonance effects, and reaction mechanisms in organic chemistry. These functional groups influence the electronic environment of aromatic and non-aromatic systems, impacting electrophilic aromatic substitution (EAS), nucleophilicity, acidity, and more. This guide will break down the definitions, examples, and effects of EWGs and EDGs to help students confidently tackle exam questions and real-world applications.
To see the impact of EWG or EDG try our Aromatic Ring Detector tool!
What Are Electron Withdrawing Groups (EWGs)?
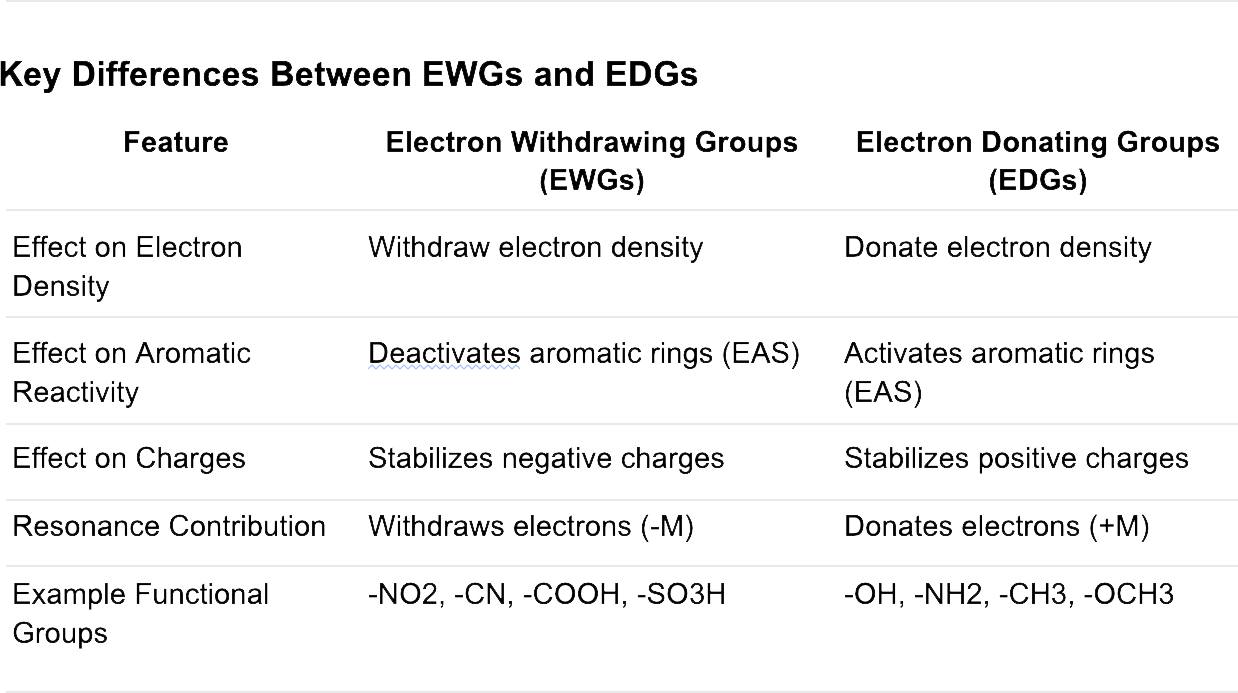
Electron withdrawing groups are functional groups that pull electron density away from a conjugated π-system or aromatic ring through resonance or inductive effects. These groups stabilize negative charges but destabilize positive charges.
Characteristics of EWGs:
Inductive Effect (-I): Withdraw electrons through sigma bonds due to electronegativity (e.g., -NO2, -CN).
Resonance Effect (-M): Withdraw electrons by resonance delocalization (e.g., -COOH, -CHO).
Increase Electrophilicity: Make the ring or molecule more susceptible to nucleophilic attack.
Decrease Reactivity in Electrophilic Aromatic Substitution (EAS): Deactivate the aromatic ring toward electrophiles.
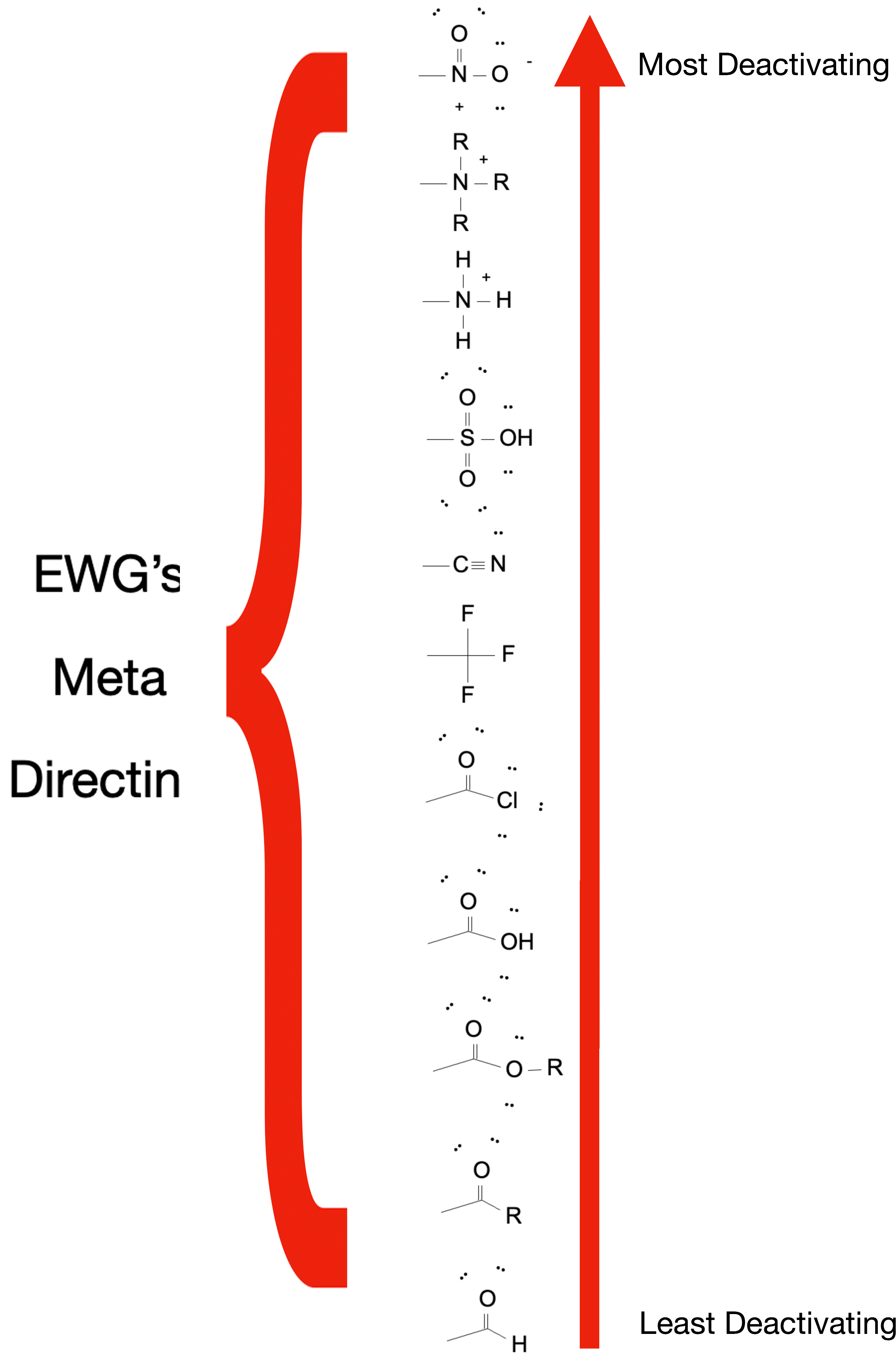
Examples of EWGs:
Nitro group (-NO2)
Carbonyl groups (-C=O, including aldehydes, ketones, carboxylic acids, esters)
Cyano group (-CN)
Halogens (-F, -Cl, -Br, -I)
Sulfonic acid group (-SO3H)
What Are Electron Donating Groups (EDGs)?
Electron donating groups are functional groups that push electron density into a conjugated π-system or aromatic ring through resonance or inductive effects. These groups stabilize positive charges but destabilize negative charges.
Characteristics of EDGs:
Inductive Effect (+I): Donate electrons through sigma bonds due to lower electronegativity or lone pairs (e.g., -CH3, -OH).
Resonance Effect (+M): Donate electrons by resonance (e.g., -OH, -NH2).
Increase Reactivity in Electrophilic Aromatic Substitution (EAS): Activate the aromatic ring toward electrophiles.
Decrease Electrophilicity: Make the ring less reactive toward nucleophiles.
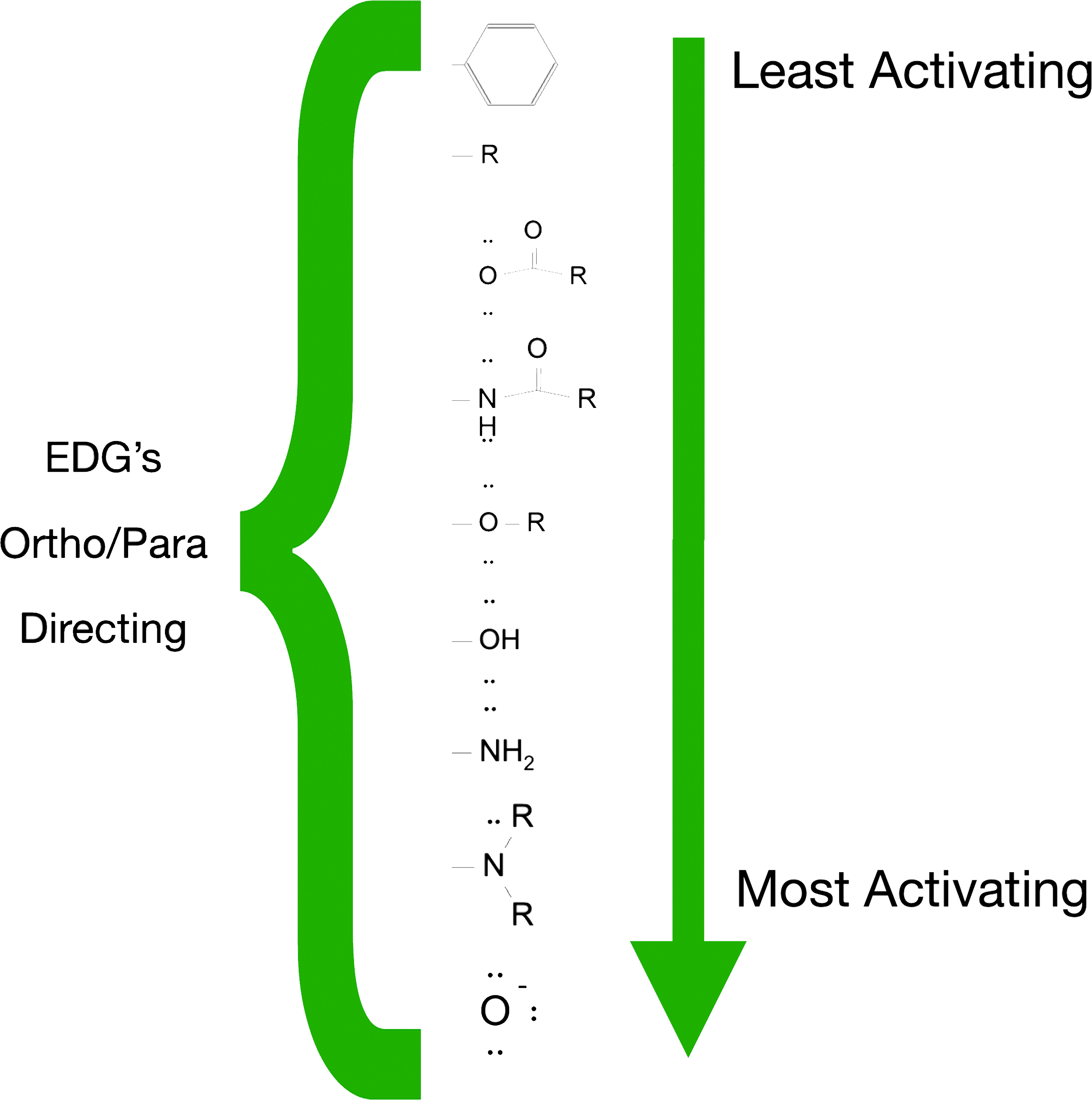
Examples of EDGs:
Hydroxyl group (-OH)
Amino group (-NH2, -NHR, -NR2)
Alkyl groups (-CH3, -R)
Alkoxy groups (-OCH3)
Common Exam Traps When Analyzing EWGs and EDGs
Misidentifying Groups:
Watch out for halogens. They are inductively withdrawing (-I) but donate through resonance (+M). Carefully assess whether resonance or inductive effects dominate.
Incorrect Positioning in EAS:
Students often forget ortho/para vs. meta directing effects. Practice identifying groups that direct substitutions correctly based on their effects.
Overlooking Charge Effects:
Groups may stabilize charges differently based on resonance versus induction. Always evaluate the group’s influence on overall electron distribution.
Ignoring Multiple Substituents:
When multiple groups are present, their combined effects must be analyzed. Dominant effects often dictate reactivity and substitution patterns.
Conclusion
Mastering the concepts of electron withdrawing groups (EWGs) and electron donating groups (EDGs) is critical for understanding organic reaction mechanisms and predicting molecular reactivity. These groups influence aromatic substitution patterns, acidity, and nucleophilicity, making them indispensable topics for organic chemistry exams and practical applications. Whether tackling exam questions, lab experiments, or designing new materials, knowing how EWGs and EDGs affect electron distribution provides a strong foundation for success in organic chemistry.