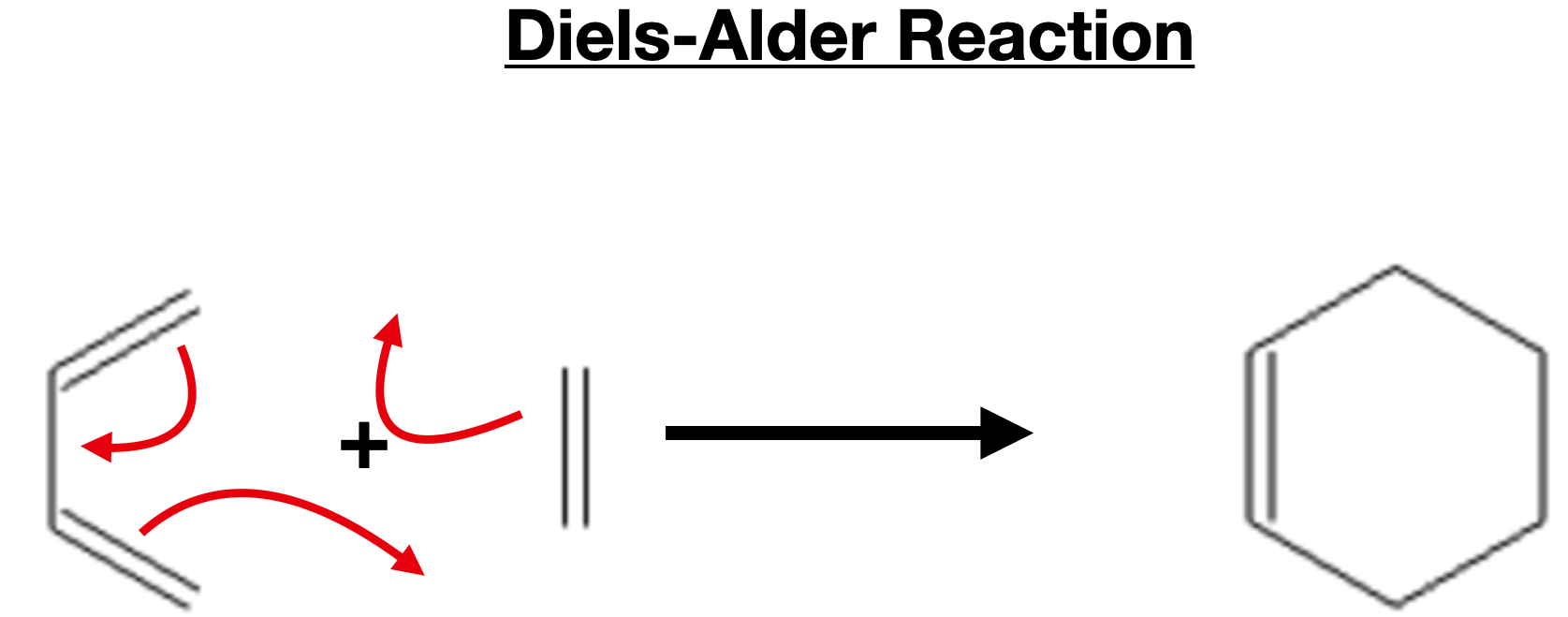
Diels-Alder Reaction
The Diels-Alder reaction, named after its discoverers Otto Diels and Kurt Alder, is one of the most powerful and versatile reactions in organic chemistry. It is a cycloaddition reaction that allows the construction of complex cyclic compounds from simple starting materials. The reaction involves the concerted formation of two new σ-bonds and leads to the formation of a six-membered ring system known as a cyclohexene. This reaction has wide-ranging applications in the synthesis of natural products, pharmaceuticals, and other important organic molecules.
Mechanism of the Diels-Alder Reaction:
The Diels-Alder reaction involves the reaction between a conjugated diene and a dienophile. A conjugated diene is a molecule with two double bonds separated by a single bond, while a dienophile is a molecule with an electron-withdrawing group that is electron-deficient. The reaction proceeds through a concerted pericyclic mechanism, where the π-electron density of the diene interacts with the electron-deficient dienophile, leading to the formation of two new σ-bonds and the cyclohexene ring system.
Regioselectivity and Stereoselectivity:
The Diels-Alder reaction exhibits remarkable regioselectivity and stereoselectivity. The regioselectivity refers to the preference for the reaction to occur at specific positions on the diene and dienophile to form a particular regioisomer. The stereoselectivity determines the formation of specific stereoisomers with respect to the geometry of the newly formed double bonds in the cyclohexene ring. These selective features allow chemists to control the formation of different products and create a diverse array of complex structures.
Regioselectivity:
Regioselectivity refers to the preference of the Diels-Alder reaction to occur at specific positions on the diene and dienophile to form a particular regioisomer. In other words, it determines which carbon-carbon double bond in the diene reacts with the dienophile to form the new sigma bonds and create the cyclohexene ring system.
The regioselectivity of the Diels-Alder reaction is governed by the electron density distribution in the reactants. Electron-rich regions in the diene tend to react with electron-deficient regions in the dienophile. Substituents on the diene and dienophile can influence the electron density and, thus, the preferred regioisomer. Electron-donating groups on the diene can increase the electron density at certain positions, favoring the formation of the regioisomer with the substituents. Conversely, electron-withdrawing groups can decrease the electron density, leading to the formation of a different regioisomer.
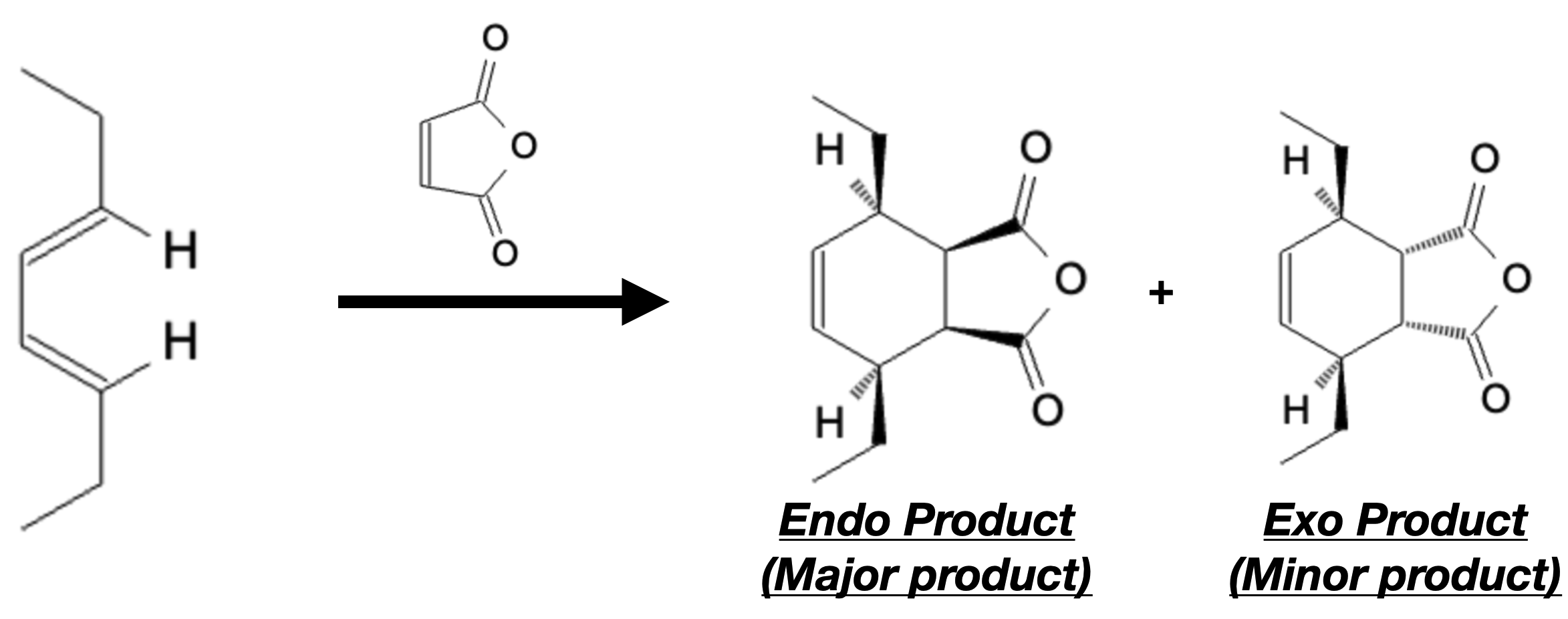
Stereoselectivity:
Stereoselectivity in the Diels-Alder reaction determines the formation of specific stereoisomers with respect to the geometry of the newly formed double bonds in the cyclohexene ring. The two primary stereoisomers produced in the reaction are endo and exo products.
The endo product is formed with the newly formed substituent on the same face as the larger bridge of the diene. In contrast, the exo product has the substituent on the opposite face. The stereochemistry of the cyclohexene ring is critical for the overall structure and properties of the final product.
Controlling Regioselectivity and Stereoselectivity:
The control of regioselectivity and stereoselectivity in the Diels-Alder reaction is essential for achieving the desired product with high yield and selectivity. Chemists can manipulate these factors by modifying the structure of the diene and dienophile. By introducing specific substituents or adjusting reaction conditions, they can guide the reaction towards the desired regioisomer and stereoisomer.
Predicting and controlling regioselectivity and stereoselectivity in the Diels-Alder reaction are essential skills for synthetic chemists. The ability to selectively access different products expands the versatility of this reaction in organic synthesis, enabling the efficient construction of complex molecules and natural products.
Substituents and Steric Effects:
Substituents on both the diene and dienophile can significantly impact the reactivity, regioselectivity, and stereoselectivity of the Diels-Alder reaction. The presence of electron-donating or electron-withdrawing groups can alter the electron density distribution in the reactants, influencing the reaction outcome.
Electron-Donating Groups:
Substituents that donate electron density to the diene or dienophile can enhance the reactivity of the reactants, making them more nucleophilic or electrophilic, respectively. Electron-donating groups on the diene can increase the electron density on the double bonds, making them more nucleophilic and prone to attacking the electron-deficient dienophile. Consequently, these groups tend to favor the formation of specific regioisomers, as the nucleophilic sites are more reactive and readily participate in the reaction.
Electron-Withdrawing Groups:
Conversely, substituents that withdraw electron density from the diene or dienophile reduce their nucleophilicity or electrophilicity. Electron-withdrawing groups on the diene decrease the electron density on the double bonds, making them less nucleophilic and less reactive. As a result, these groups may lead to the formation of different regioisomers in the Diels-Alder reaction compared to electron-donating substituents.
Steric Effects:
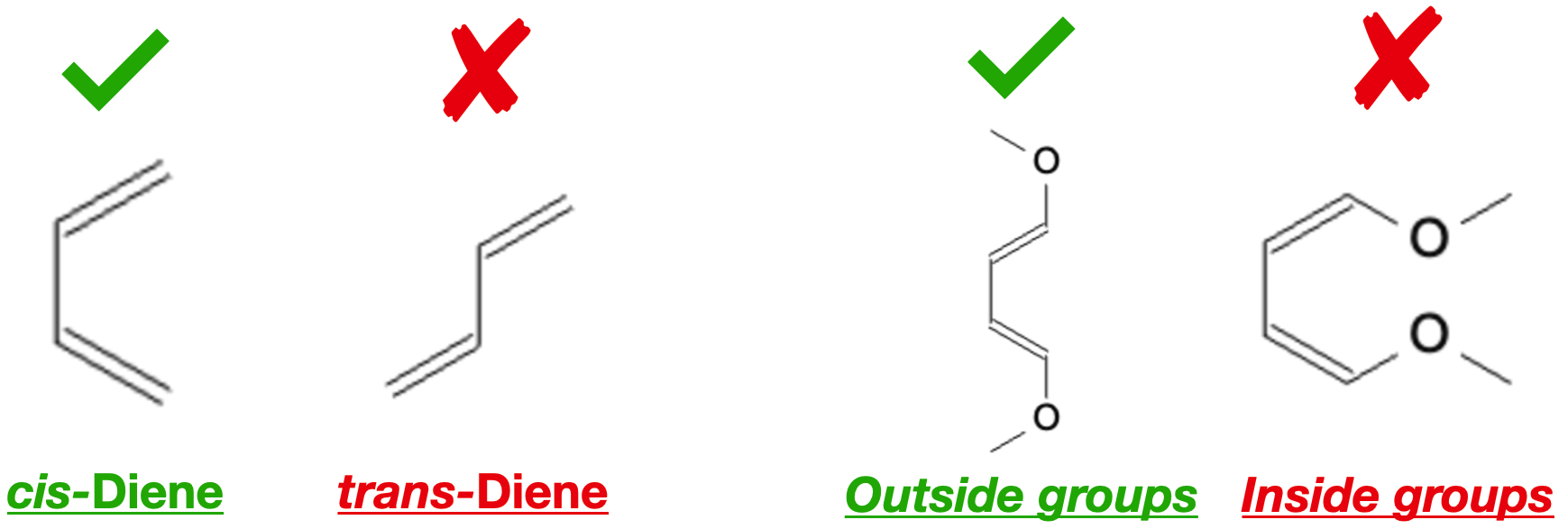
Steric effects play a crucial role in the Diels-Alder reaction as well. When bulky substituents are present on either the diene or dienophile, they can hinder the approach of the reactants to each other, affecting both regioselectivity and stereoselectivity. For instance, large substituents can block the formation of certain regioisomers by sterically hindering the formation of the desired transition state.
The diene should always be in a cis configuration, and the groups attached to the diene should be on the outside, not inside:
Summary
In conclusion, the Diels-Alder reaction is a powerful and versatile tool in organic synthesis. Its unique ability to form complex cyclic structures from simple starting materials, coupled with its regioselectivity and stereoselectivity, has made it an indispensable reaction in modern organic chemistry. The Diels-Alder reaction has revolutionized the field of synthetic chemistry, enabling the efficient and controlled construction of intricate molecular architectures and contributing to the development of novel pharmaceuticals and materials. As research continues to uncover new variations and applications of the Diels-Alder reaction, it is poised to remain a cornerstone of organic synthesis for years to come.
Test Your Knowledge
How do electron-donating and electron-withdrawing substituents on the diene and dienophile influence the regioselectivity of the Diels-Alder reaction? Provide an example reaction to illustrate the concept.
Explain the impact of steric effects on the stereoselectivity of the Diels-Alder reaction. Provide an example where steric hindrance dictates the formation of a specific stereoisomer, and describe how the reaction outcome can be controlled by modifying the reactant's structure.