Cycloalkane Conformations (Chair, Boat, Ring Flip)
Cycloalkane Conformations (Chair, Boat, Ring Flip)
Cycloalkane shapes set their strain and stability. Ring strain combines angle, torsional, and steric terms. Cyclopropane/cyclobutane are strained; cyclopentane puckers to relieve eclipsing. Cyclohexane adopts low-strain forms, with the chair dominating.
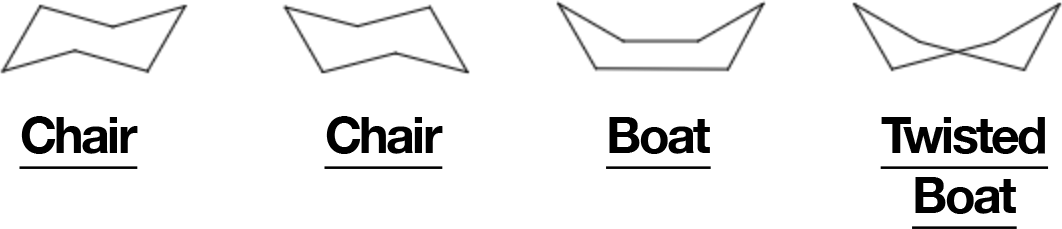
Chair vs Boat (and Twist-Boat)
- Chair: ~109.5° angles, all bonds staggered → minimal strain.
- Boat: eclipsing bonds + flagpole clashes at C1/C4 → much higher energy.
- Twist-boat: relieves some boat strain but still above chair (~5.5 kcal/mol).
Axial vs Equatorial; Ring Flips
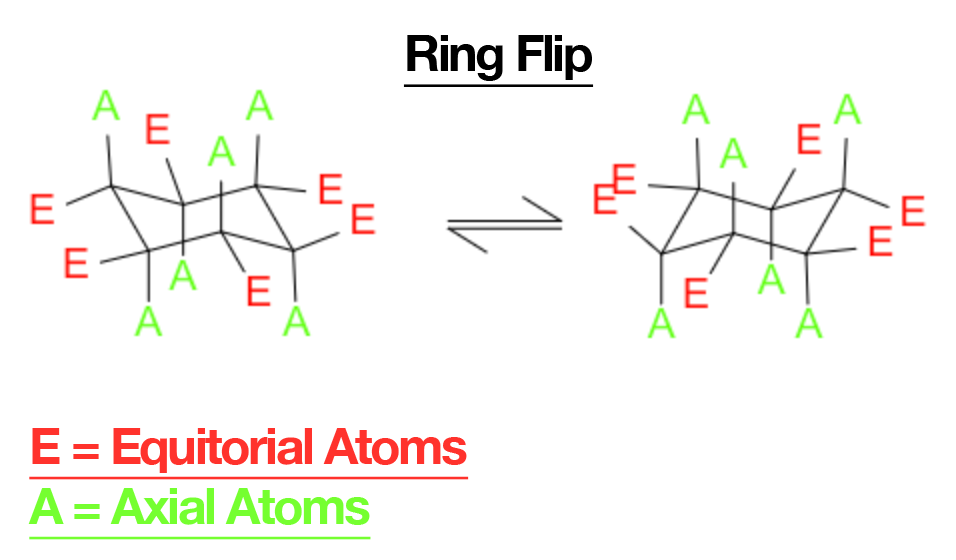
- Each chair carbon has axial (up/down) and equatorial (outward) positions.
- Ring flip interconverts chairs: axial ⇄ equatorial; “up” stays up, “down” stays down.
- Substituents prefer equatorial to avoid 1,3-diaxial (gauche-like) interactions. Example: equatorial Me in methylcyclohexane is ~1.7 kcal/mol more stable than axial.
Key Takeaways
- Chairs are far more stable than boats; twist-boat is intermediate.
- Ring flips swap axial/equatorial; bulkier groups seek equatorial positions.
- Predicting dominant conformers guides expected equilibria and reactivity in substituted cyclohexanes.