Conformational Isomers of Acyclic Alkanes (Newman Projections)
Conformational Isomers of Acyclic Alkanes (Newman Projections)
Single-bond rotation produces different conformations, but not all are equally stable. Newman projections let you look down a C–C bond to compare torsional strain and steric clashes in acyclic alkanes.
Newman Projections and Dihedral Angles
- View straight down a C–C bond: front carbon as a dot, back carbon as a circle.
- Dihedral angle: angle between a front substituent and a back substituent.
- Staggered (≈60° offsets) minimizes torsional strain; eclipsed (0°) maximizes it.
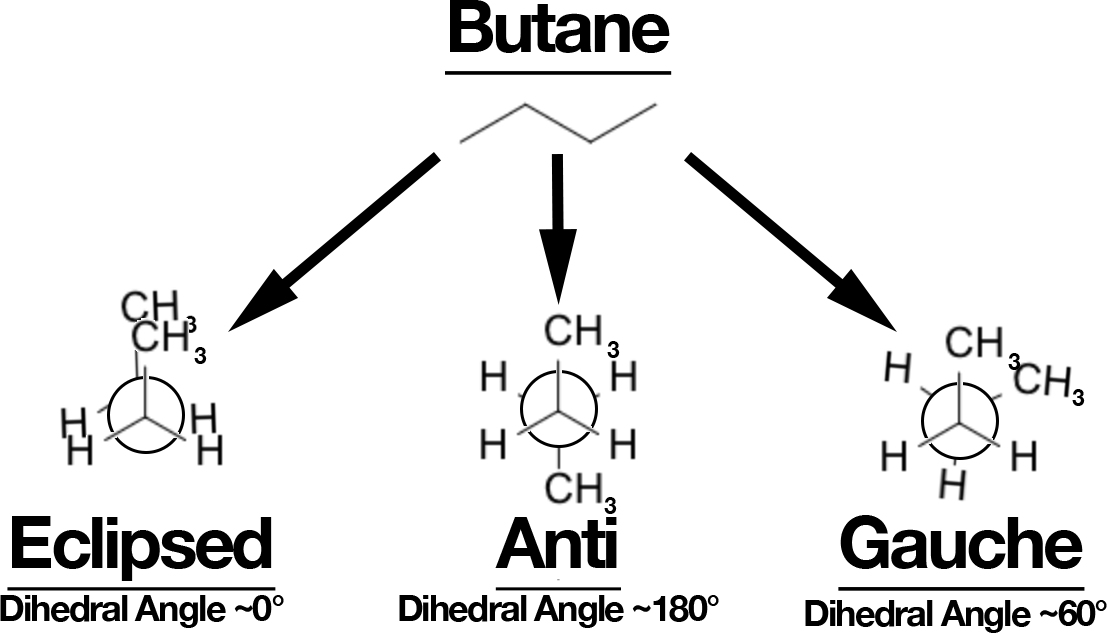
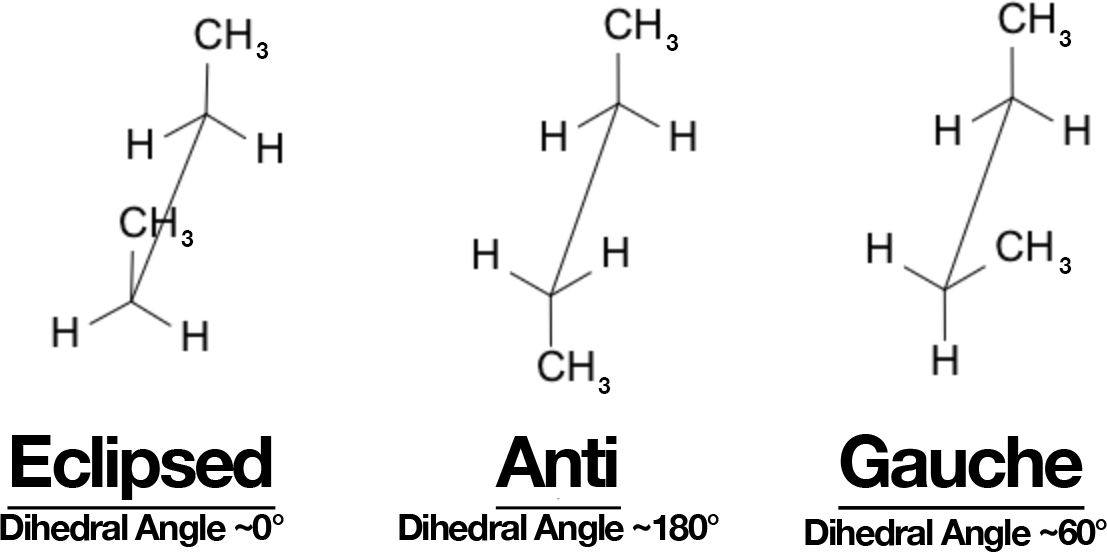
Butane: Anti vs Gauche (and Eclipsed)
- Look down C2–C3.
- Anti (staggered, 180°): CH₃ groups opposite → lowest energy.
- Gauche (staggered, 60°): CH₃ groups closer → ~0.9 kcal/mol higher (steric bump).
- Eclipsed: highest energy, especially when CH₃ eclipses CH₃ (torsional + steric).
Rotation energy profile: anti (min) → eclipsed (H/CH₃) → gauche → eclipsed (CH₃/CH₃, max).
Why It Matters
Preferred conformations dictate dominant shapes, affect reaction approach trajectories, and rationalize observed selectivity (e.g., anti-periplanar requirements in E2).
Summary
Use Newman projections to spot torsional and steric strain. Staggered beats eclipsed; in butane, anti is most stable, gauche slightly higher, eclipsed forms are transient. Mastering these views helps predict favored shapes and reactivity.