Acid/Base Fundamentals
Acid/Base Fundamentals (Arrhenius, Brønsted–Lowry, Lewis)
Acids and bases can be framed three ways: Arrhenius (H⁺/OH⁻ in water), Brønsted–Lowry (proton donors/acceptors), and Lewis (electron-pair acceptors/donors). Picking the right definition lets you describe acid–base behavior in water, non-aqueous solvents, and even purely Lewis interactions.
- Arrhenius: H⁺/OH⁻ in Water
- Brønsted–Lowry: Proton Transfers
- Lewis: Electron-Pair Interactions
- Summary
Arrhenius: H⁺/OH⁻ in Water
- Arrhenius acid: produces H⁺ (as H₃O⁺) in water; e.g., HCl → H₃O⁺ + Cl⁻.
- Arrhenius base: produces OH⁻ in water; e.g., NaOH → Na⁺ + OH⁻.
- Useful for aqueous systems only.

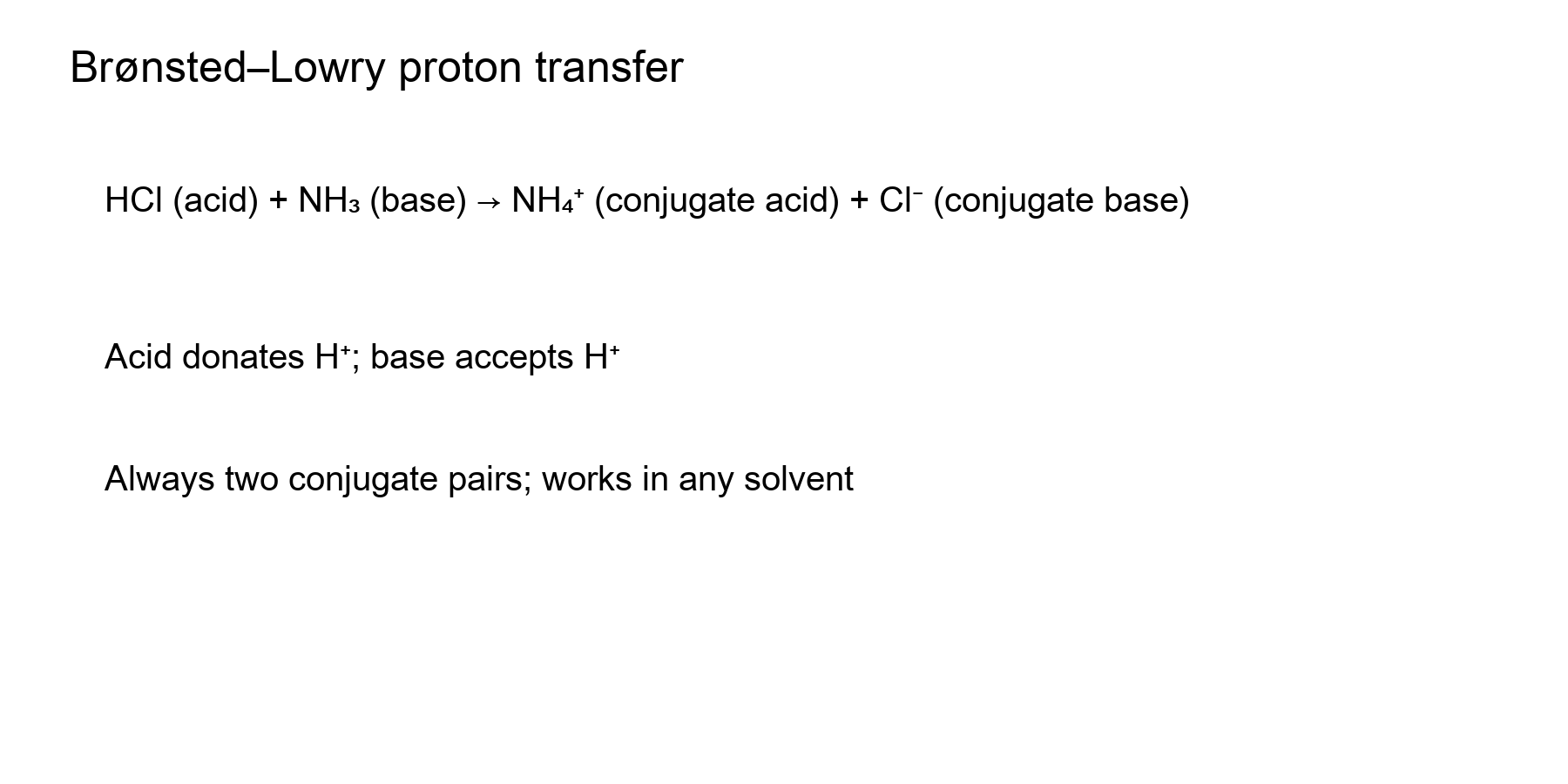
Brønsted–Lowry: Proton Transfers
- Acid = proton donor; base = proton acceptor (works in any medium).
- Example: HCl donates H⁺ to NH₃ → NH₄⁺ (conjugate acid) + Cl⁻ (conjugate base).
- Always two conjugate pairs; acid/base strength is inversely related to their conjugates.
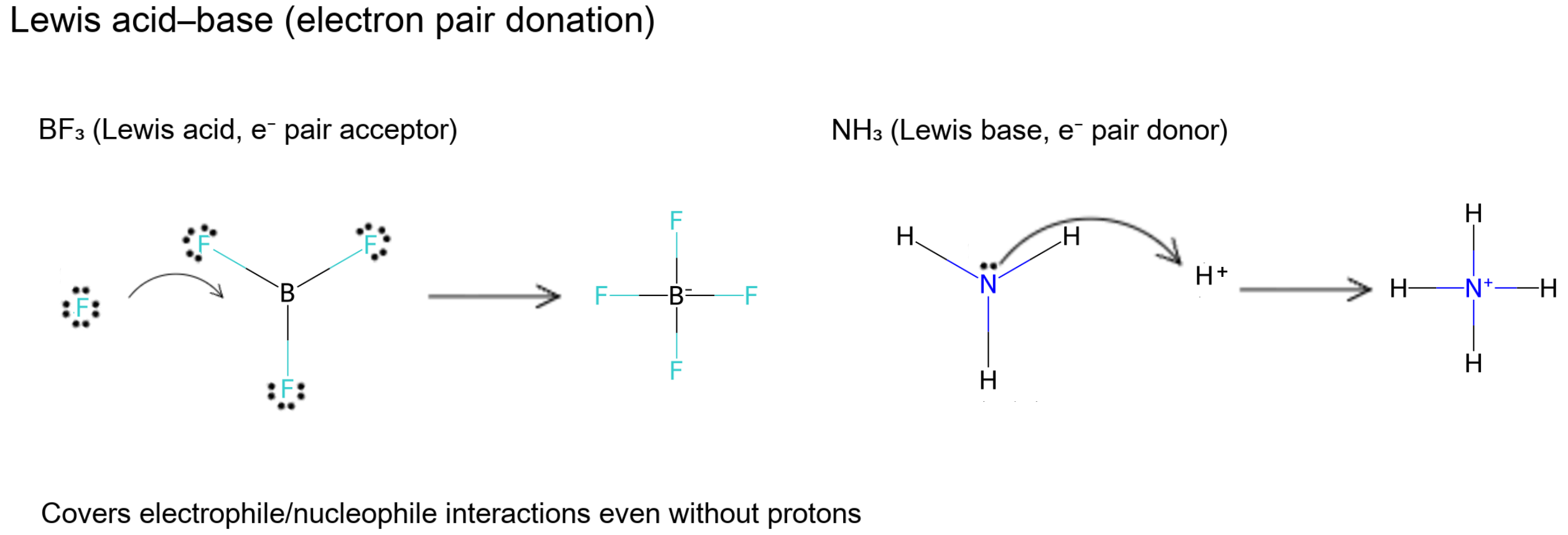
Lewis: Electron-Pair Interactions
- Lewis acid = electron-pair acceptor; Lewis base = electron-pair donor.
- Captures reactions without proton transfer (e.g., BF₃ accepting a lone pair from NH₃).
- Mirrors electrophile (acid) vs nucleophile (base) language.
Summary
- Arrhenius covers aqueous H⁺/OH⁻ generation; Brønsted–Lowry generalizes proton transfers to any medium.
- Lewis focuses on electron pairs, covering electrophile–nucleophile interactions with or without protons.
- Conjugate acid/base strengths are inversely related; choose the broadest definition that fits the reaction.