Conjugate Acids and Bases
Conjugate Acids and Bases
Acids and bases always form conjugate pairs that differ by exactly one proton. Tracking these pairs helps predict which direction an acid–base equilibrium will fall and how strong each species is.
Identifying Conjugate Pairs
- Conjugate base: remove H⁺ from an acid and adjust the charge. Example: HCl → Cl⁻.
- Conjugate acid: add H⁺ to a base and adjust the charge. Example: NH₃ + H⁺ → NH₄⁺.
- Every acid–base reaction shows two conjugate pairs: the acid/conjugate base and the base/conjugate acid.
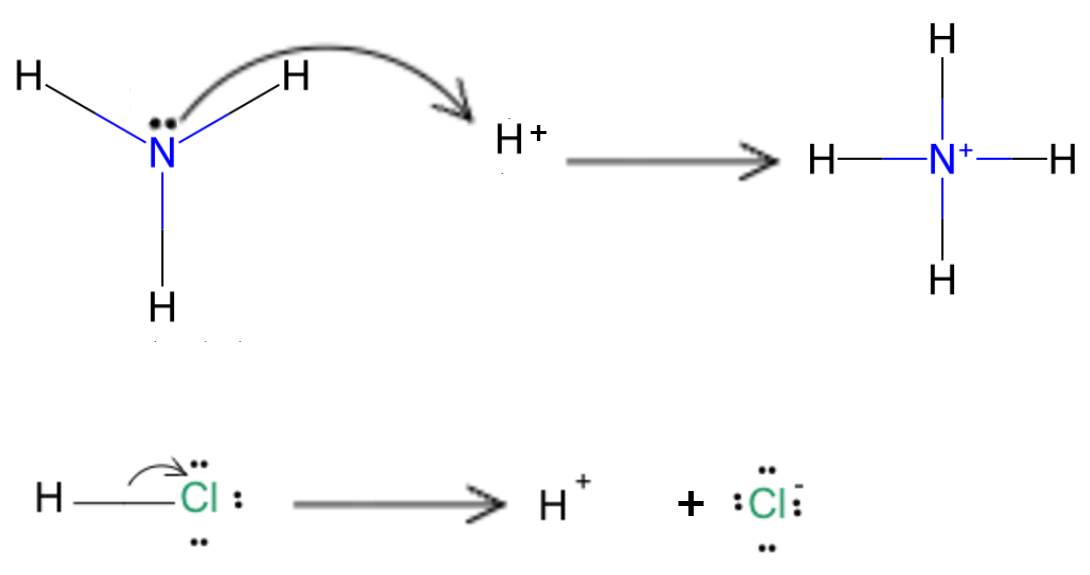
Arrow from HCl to Cl⁻ + H⁺, and from NH₃ + H⁺ to NH₄⁺ (conjugate pairs).
Strength Relationships
- Strong acid ⇄ very weak conjugate base (it has little tendency to regain H⁺).
- Weak acid ⇄ stronger conjugate base (more likely to accept H⁺).
- Each unit change in pKₐ is a 10× difference in acid strength, so comparing conjugate pairs approximates eq direction.
| Acid (common / IUPAC) | Structure | pKₐ | Conjugate base structure | Conjugate base name |
|---|---|---|---|---|
| Acetic acid / Ethanoic acid | 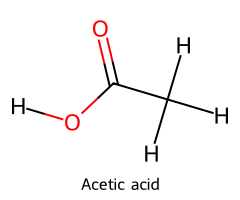 | ≈ 4.8 | 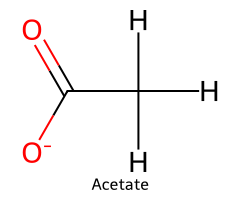 | Acetate ion |
| Methylammonium / Methanaminium | 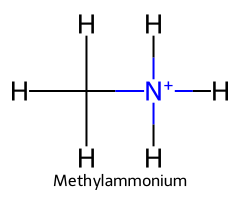 | ≈ 10.6 | 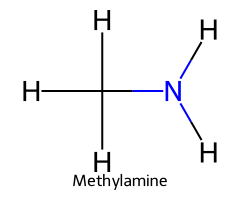 | Methylamine |
| Phenol / Phenol | 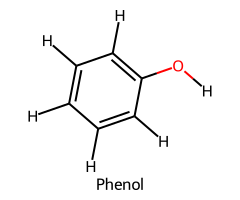 | ≈ 10 | 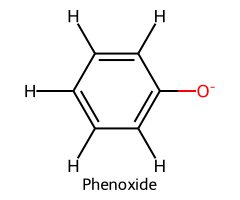 | Phenoxide |
| Ethanethiol / Ethane-1-thiol | 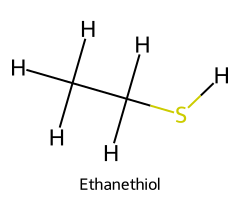 | ≈ 10.6 | 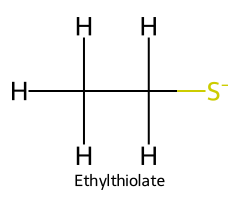 | Ethylthiolate |
| Water / Oxidane | 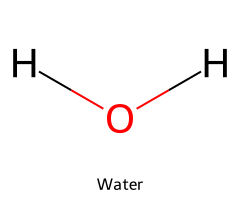 | 15.7 | 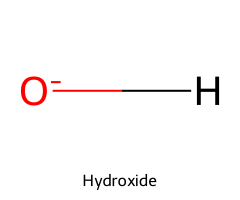 | Hydroxide |
| Ethanol / Ethan-1-ol | 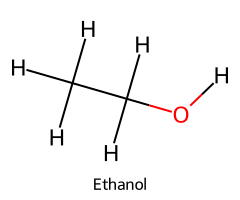 | ≈ 16 | 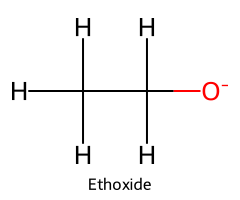 | Ethoxide |
| Acetone (α-C–H) / Propan-2-one | 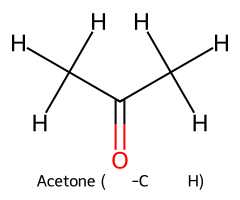 | ≈ 19–20 | 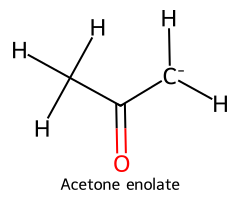 | Acetone enolate |
| Acetylene / Ethyne | 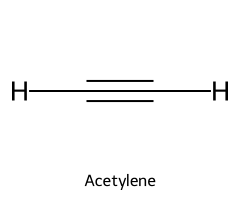 | ≈ 25 | 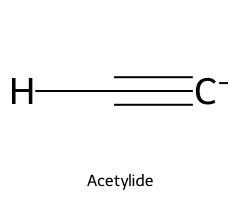 | Acetylide |
| Ammonia / Azane | 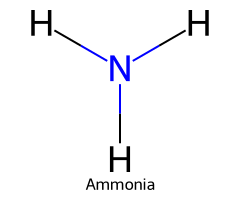 | ≈ 38 | 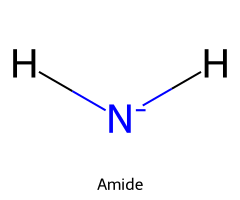 | Amide |
| Methane / Methane | 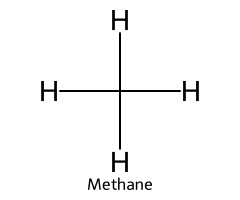 | > 50 | 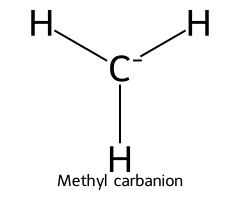 | Methyl carbanion |
Summary
- Conjugate pairs differ by one proton; acid/conjugate base on one side, base/conjugate acid on the other.
- Track both conjugate pairs to understand which direction a reaction goes.
- Strong acids pair with weak conjugate bases; weak acids pair with relatively stronger bases.