Alkene Structure and Nomenclature
Alkene Structure and Nomenclature
Alkenes are unsaturated hydrocarbons with at least one C=C double bond (general formula CnH2n). Each alkene carbon is sp², giving ~120° bond angles and a planar, rotation-restricted double bond that drives reactivity and stereochemistry.
What Defines an Alkene
- Acyclic alkenes follow CnH2n; rings adjust hydrogen count.
- The double bond = one σ + one π bond; the π bond is electron rich → alkenes are nucleophilic and undergo electrophilic addition.
- No free rotation about C=C → geometric isomerism (cis/trans or E/Z) when each sp² carbon bears two different groups.
IUPAC Naming Rules
- Parent chain includes the double bond; suffix -ene.
- Lowest locant for the double bond; locant placed before “-ene” (e.g., hex-2-ene).
- Multiple C=C: “-diene”, “-triene”, etc., with all locants (e.g., penta-1,4-diene).
- Substituents named/numbered like alkanes; number from the end nearest the C=C.
- Rings: “cyclo” + locant 1,2 for the C=C; number substituents for lowest set.
- Geometric tags when applicable: cis/trans or E/Z (CIP priority).
Examples (acyclic and cyclic)
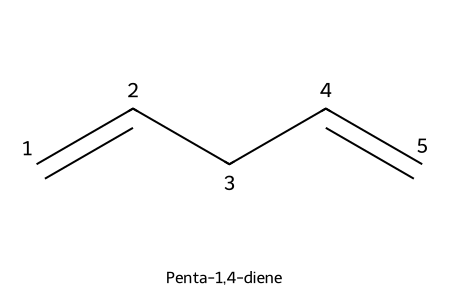
IUPAC: penta-1,4-diene
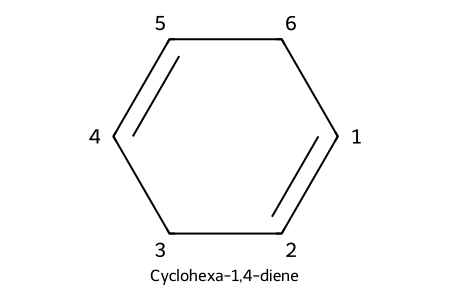
IUPAC: cyclohexa-1,4-diene
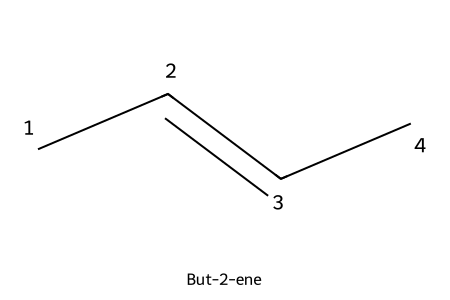
IUPAC: but-2-ene
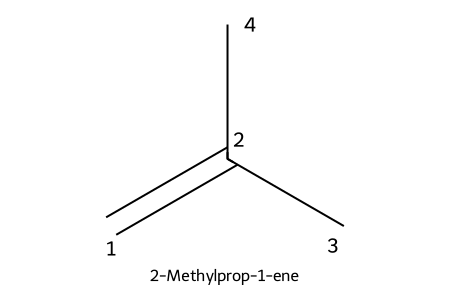
IUPAC: 2-methylprop-1-ene
IUPAC: cyclopent-1-ene
Summary
- Alkenes are sp², π-rich, and conform to CnH2n (acyclic).
- Name by longest chain containing C=C, lowest locant for the double bond, “-ene” suffix, and E/Z or cis/trans when needed.
- Planarity and restricted rotation set up geometric isomerism and guide reactivity (electrophilic additions).