E/Z and Cis/Trans Alkene Nomenclature
E/Z and Cis/Trans Alkene Nomenclature
Double bonds lock substituents in place, creating geometric isomers. Two systems describe them: cis/trans for simple cases and E/Z (CIP-based) for all alkenes.
Cis/Trans (Simple Cases)
- Works when each alkene carbon has one substituent in common (often H).
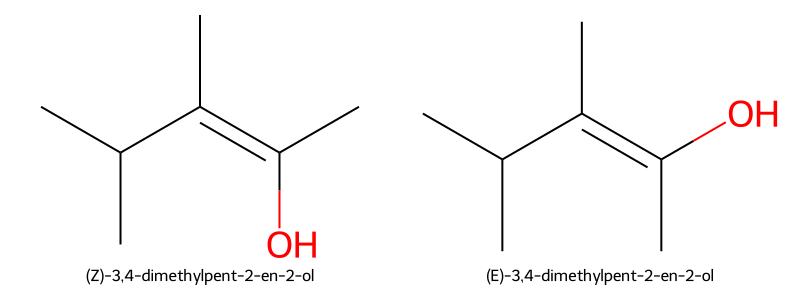
- Example: (Z)-3,4-dimethylpent-2-en-2-ol (higher groups same side) vs (E)-3,4-dimethylpent-2-en-2-ol (higher groups opposite); polarity differs → different boiling points.
- Becomes ambiguous when all four substituents differ or the double bond is tri-/tetrasubstituted.
E/Z (Universal System)
Apply CIP priorities on each alkene carbon:
- Rank the two substituents on carbon 1 (higher atomic number wins; apply CIP tie-breakers).
- Rank the two on carbon 2.
- Compare the higher-priority group on each carbon:
- Z (zusammen, together): high-priority groups on the same side.
- E (entgegen, opposite): high-priority groups on opposite sides.
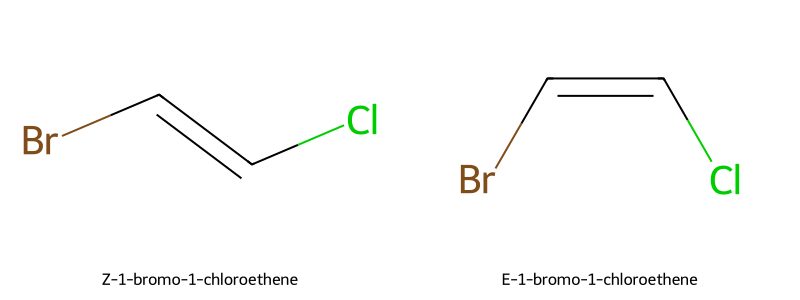
Example: 1-bromo-1-chloroethene → Br > H on one carbon, Cl > H on the other; Br/Cl together = Z, opposite = E.
Mnemonic: think “Z = zee zame zide” (Z = same side), and E as “elsewhere” (opposite).
When to Use Which
- Use cis/trans only when identical substituents make it unambiguous.
- Default to E/Z for any substituted alkene to avoid ambiguity and capture tri-/tetrasubstituted cases.

Summary
Cis/trans naming is handy for simple alkenes; E/Z (with CIP priorities) works universally. Identify the two highest-priority substituents—same side gives Z, opposite gives E—ensuring unambiguous stereochemical names.