Resonance Stabilization of Conjugate Bases
Resonance Stabilization of Conjugate Bases
Resonance that spreads negative charge stabilizes a conjugate base and makes its parent acid much stronger. Carboxylic acids and phenols are classic examples; analogous alcohols lack this delocalization.
Carboxylic Acids vs. Alcohols
- Acetic acid (pKₐ ~4.8) vs ethanol (pKₐ ~16): acetate delocalizes charge over two oxygens; ethoxide localizes it on one oxygen.
- Resonance makes acetate far more stable; acetic acid is ~10¹¹ times more acidic than ethanol.
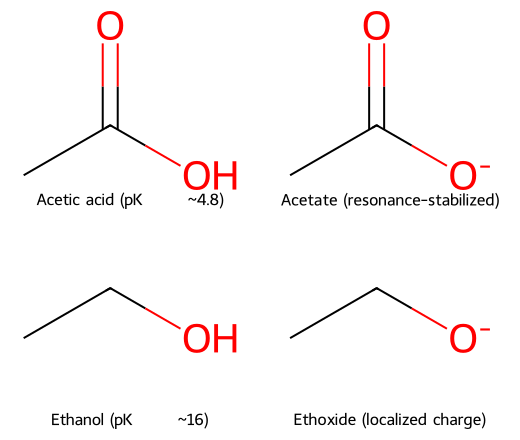
Acetate (resonance-stabilized) vs. ethoxide (localized charge).
Phenols and Other Resonance Effects
- Phenol (pKₐ ≈ 10) vs cyclohexanol (~16–18): phenoxide delocalizes charge into the aromatic ring; cyclohexoxide cannot.
- Any conjugate base next to a carbonyl, aromatic ring, or conjugated system can delocalize the anion, boosting acidity.
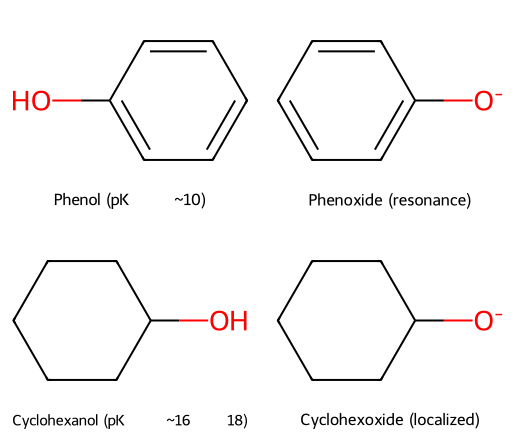
Phenoxide spreads negative charge into the ring; cyclohexoxide cannot.
Summary
- More resonance forms → more charge delocalization → stronger acid.
- Carboxylates and phenoxides are stabilized by resonance; corresponding alcohols are not.
- Look for conjugate bases that can share negative charge across π systems to predict higher acidity.