pKₐ Values and Acidity Trends
pKₐ Values and Acidity Trends
pKₐ (−log Kₐ) quantifies acid strength: lower pKₐ → stronger acid. Each pKₐ unit is a 10× change, so functional groups span huge ranges. Here are key benchmarks and the structural factors that stabilize conjugate bases.
Benchmark pKₐ Values
Using the same visual format as the conjugate-pairs article, here are common acids with pKₐ and their conjugate bases.
| # | Acid | Structure | pKₐ (water) | Conjugate base | Conjugate base (name) |
|---|---|---|---|---|---|
| 1 | Acetic acid / Ethanoic acid | 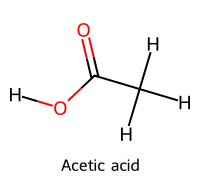 | 4.76 | 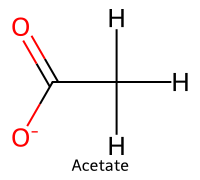 | Acetate |
| 2 | Phenol / Phenol | 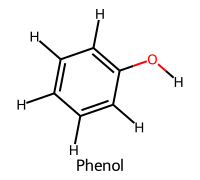 | ≈10.0 | 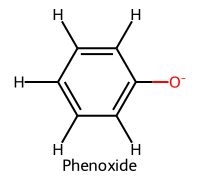 | Phenoxide |
| 3 | Methylammonium / Methanaminium | 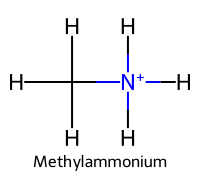 | ≈10.6 | 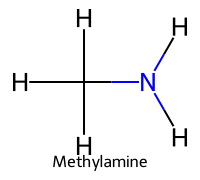 | Methylamine |
| 4 | Ethanethiol / Ethane-1-thiol | 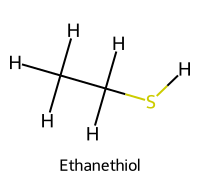 | ≈10.6 | 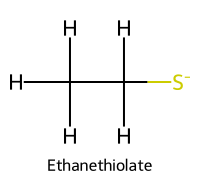 | Ethanethiolate |
| 5 | Ethanol / Ethan-1-ol | 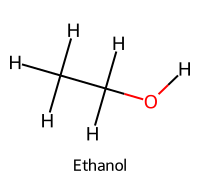 | ≈16 | 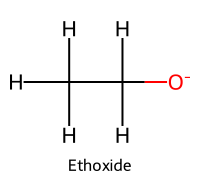 | Ethoxide |
Bases and Conjugate-Acid pKₐ Benchmarks
| # | Base | Structure | Conjugate acid pKₐ (water) | Conjugate acid | Conjugate acid (name) |
|---|---|---|---|---|---|
| 1 | Acetate | 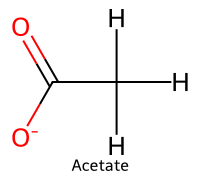 | 4.76 | 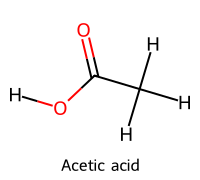 | Acetic acid |
| 2 | Pyridine | 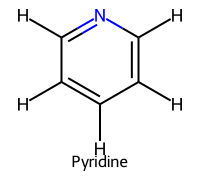 | ≈5.2 | 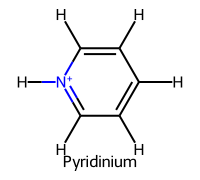 | Pyridinium |
| 3 | Ammonia | 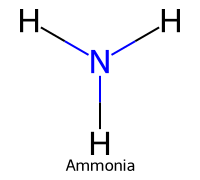 | ≈9.25 | 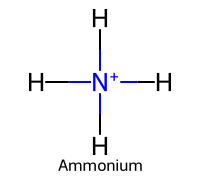 | Ammonium |
| 4 | Methylamine | 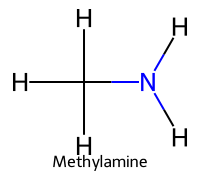 | ≈10.6 | 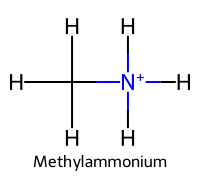 | Methylammonium |
| 5 | Ethoxide | 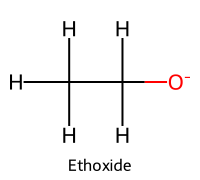 | 15.9 | 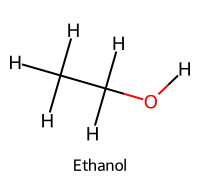 | Ethanol |
Factors That Increase Acidity (Stabilize Conjugate Base)
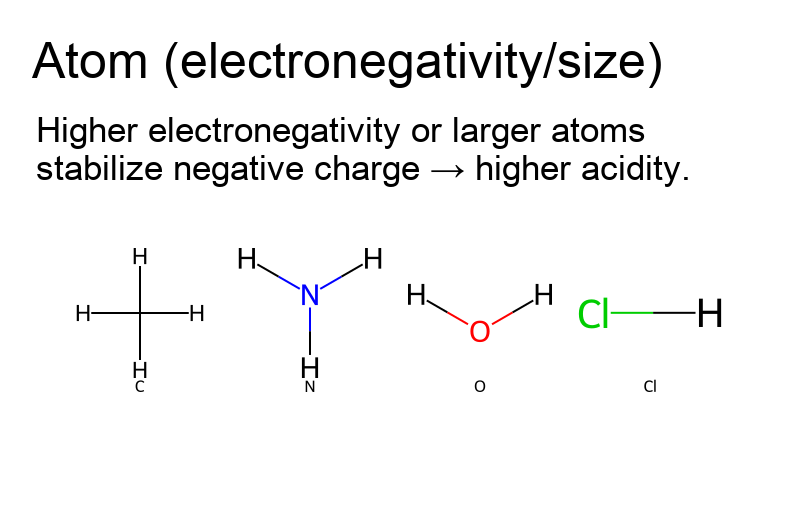
- Atom (electronegativity/size): More electronegative or larger atoms better stabilize charge.
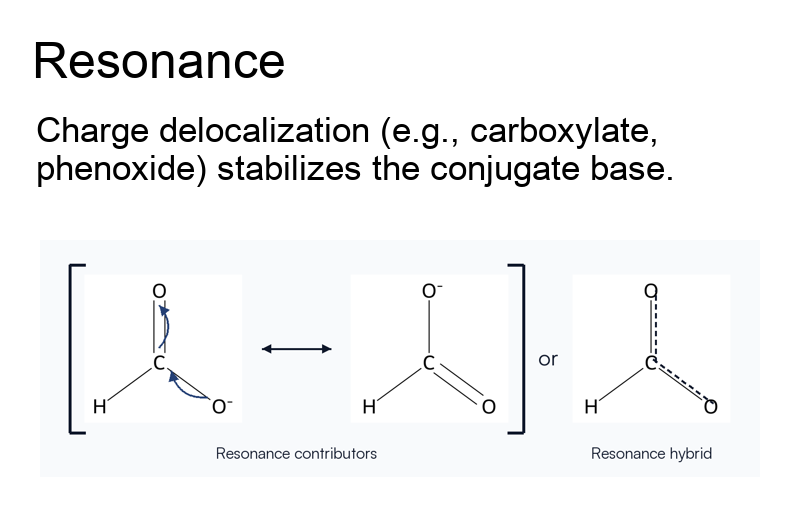
- Resonance: Delocalizing negative charge (e.g., carboxylates) greatly boosts acidity.
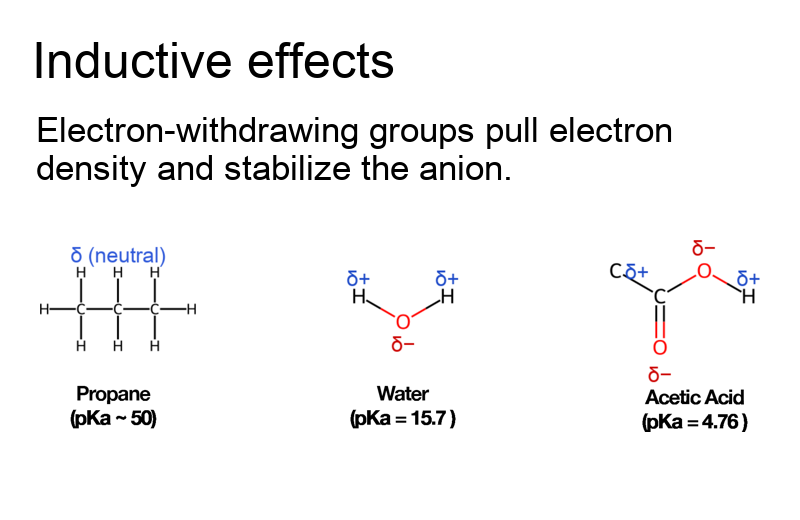
- Inductive effects: Electron-withdrawing groups pull electron density and stabilize the anion.
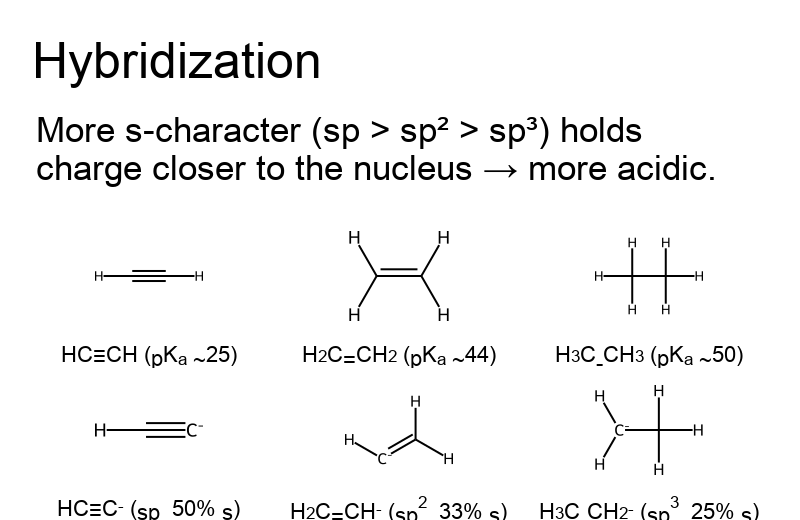
- Hybridization: More s-character (sp > sp² > sp³) stabilizes the anion and raises acidity.
Summary
- pKₐ is logarithmic; small shifts mean big strength changes.
- Acidity tracks with conjugate-base stability: atom effects, resonance, induction, and hybridization all matter.
- Use benchmarks to triage acidity (strong acids ~ −7, carboxylic ~5, alcohols ~16, amines ~38, alkanes ~50).