Chirality and Enantiomers
Chirality and Enantiomers
Chirality is molecular “handedness.” A chiral molecule is not superimposable on its mirror image, giving rise to enantiomers—pairs of mirror-image stereoisomers.
Chiral Centers and Handedness
- A tetrahedral carbon with four different substituents is a classic chiral center.
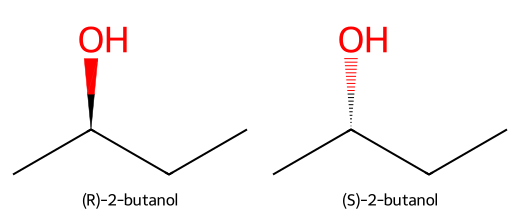
- Example: 2-butanol has a chiral carbon bonded to CH₃, CH₂CH₃, OH, and H → two enantiomers (R and S).
- Chirality is a whole-molecule property; internal symmetry can make a molecule achiral even with stereocenters.
Properties of Enantiomers
- Identical in achiral environments (bp, mp, NMR).
- Opposite optical rotations: one rotates plane-polarized light clockwise (+), the other counterclockwise (–).
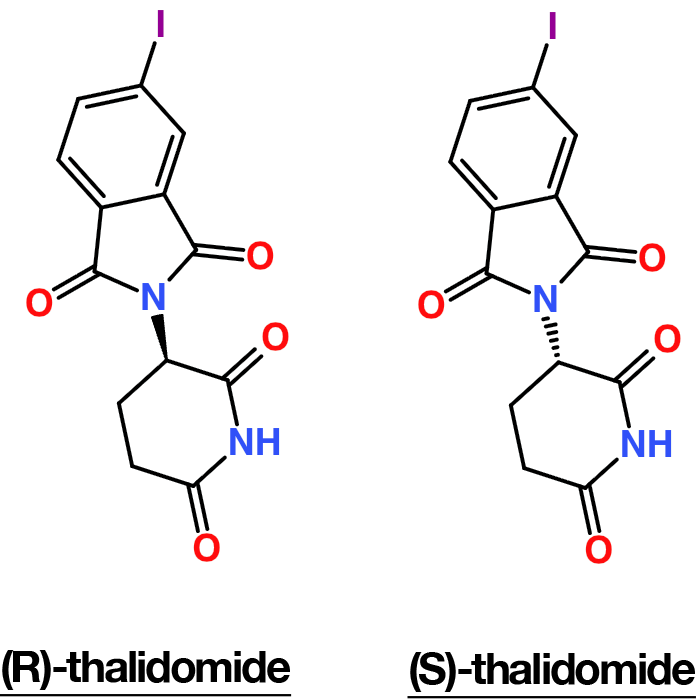
- In chiral environments (enzymes, receptors), enantiomers can behave differently (e.g., thalidomide enantiomers; carvone odors).
Thalidomide is a classic example of how enantiomers can have dramatically different biological effects in a chiral environment. In vitro and in animal studies, the (R)-enantiomer is associated primarily with the desired sedative / antiemetic and immunomodulatory effects, whereas the (S)-enantiomer is responsible for severe teratogenicity, including limb malformations in developing embryos. Historically, thalidomide was marketed as a racemate in the late 1950s–early 1960s; widespread use in pregnant patients led to thousands of cases of congenital defects, making it one of the most prominent drug disasters in medicinal history.
Recognizing and Drawing Enantiomers
- Lack of internal symmetry → likely chiral.
- Draw one form and its mirror image (invert wedges/dashes); no rotation will superimpose them.
- Racemic mixtures contain both enantiomers; pure enantiomers interact distinctly with chiral media.
Summary
Chiral molecules cannot be superimposed on their mirror images. Enantiomers share most properties but differ in optical rotation and interactions with chiral systems. Spotting chiral centers and symmetry lets you predict when enantiomers—and their divergent behaviors—matter.