Assigning R and S Configuration
Assigning R and S Configuration
Absolute configuration (R / S) is how we label the 3D arrangement of groups around a chiral center. Two molecules with the same connectivity but opposite R/S configuration at a stereocenter are enantiomers—non‑superimposable mirror images that are identical in most physical properties but rotate plane‑polarized light in equal and opposite directions and often behave differently in chiral environments (enzymes, receptors, chiral catalysts).
This lesson shows how to see chirality in 3D and then use the Cahn–Ingold–Prelog (CIP) rules to assign R and S.
1. Seeing Chirality in 3D
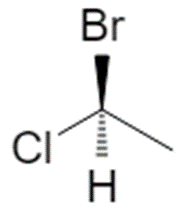
1.1 Example: 1‑bromo‑1‑chloroethane
Start from the flat (2D) drawing of 1‑bromo‑1‑chloroethane:
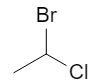
On paper this just looks like “Br and Cl on the same carbon”. In 3D, that central carbon is attached to four different substituents (Br, Cl, CH₃, H), so it is a chiral center and can exist as two configurations, R and S. :contentReference[oaicite:2]{index=2}
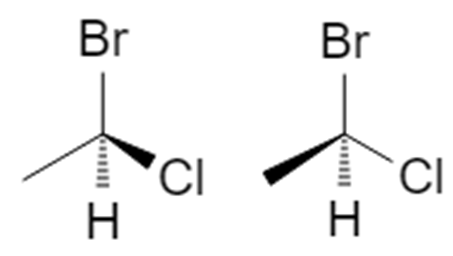
3D wedge‑and‑dash models of the two enantiomers:
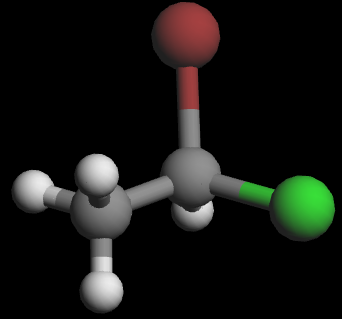
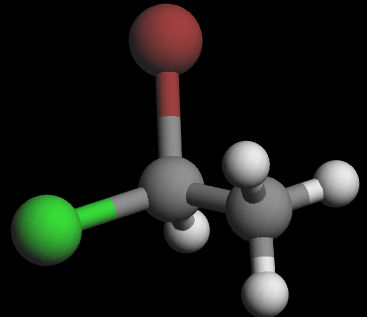
Visually, they are mirror images. To show that they are non‑superimposable, try lining them up:
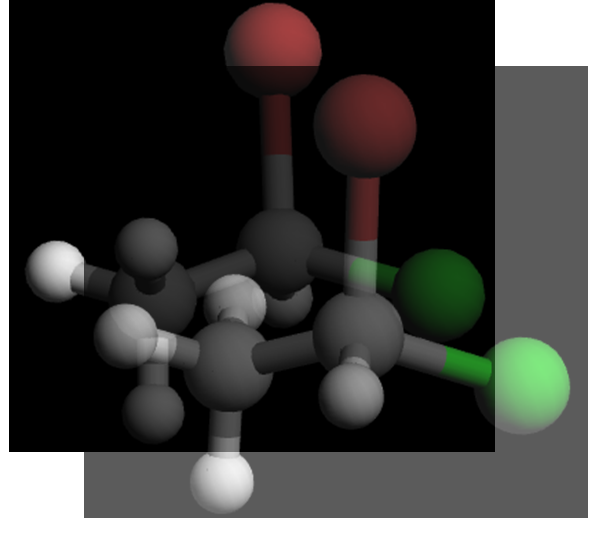
Even after rotating, you can’t make all four groups overlap at once: when Br, Cl, and CH₃ are aligned, the H in one structure points forward while in the other it points backward. That’s the hallmark of enantiomers.
We call these two molecules:
- (1R)-1-bromo-1-chloroethane
- (1S)-1-bromo-1-chloroethane
Additional 3D views of the same R and S structures:

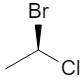

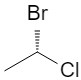
2. Chiral vs Achiral: 2‑bromobutane
A chiral center (stereocenter) in organic chemistry is usually an sp³ carbon bonded to four different groups.
Consider 2‑bromobutane:
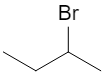
At C‑2, the carbon is attached to:
- Br
- H (implicit)
- CH₃– (methyl)
- CH₃CH₂– (ethyl)
All four are different, so C‑2 is chiral. The two enantiomers are:
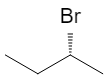

Again, these are mirror images that are not superimposable. They have the same boiling point, melting point, NMR, and most other properties in an achiral environment, but rotate plane‑polarized light in opposite directions and often show different behavior in biological systems.
3. CIP Priority Rules (Cahn–Ingold–Prelog)
To assign R or S at a chiral center, we must rank the four substituents in order of priority using the CIP rules.
Rule 1 – Atomic number
- Higher atomic number = higher priority.
- Example: Br (35) > Cl (17) > C (6) > H (1).
Rule 2 – First point of difference
If two substituents start with the same atom (e.g., both carbons), move one bond out and compare the list of atoms attached. The first difference in atomic number decides.
Rule 3 – Multiple bonds
Treat a double bond as if that atom were connected to the same neighbor twice; a triple bond as if connected three times (for priority purposes only).
Rule 4 – Isotopes
Heavier isotopes have higher priority (e.g., D > H).
Example: 1‑bromo‑1‑chloroethane priorities
| Substituent | Attached atom | Atomic number | CIP priority |
|---|---|---|---|
| Br | Br | 35 | 1 |
| Cl | Cl | 17 | 2 |
| CH₃ (methyl) | C | 6 | 3 |
| H | H | 1 | 4 |
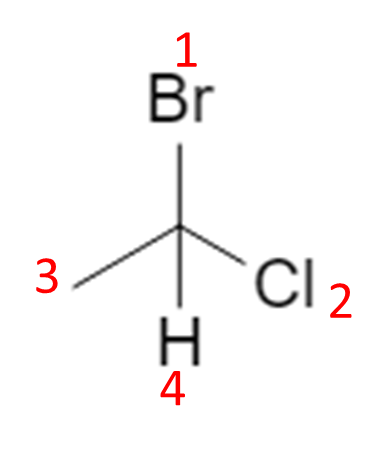
4. Four-Step Workflow to Assign R / S
Once priorities 1–4 are assigned, use this workflow:
- Rank substituents (1–4) with CIP rules.
- Orient the molecule so that the lowest priority (4) points away from you (on a dashed wedge).
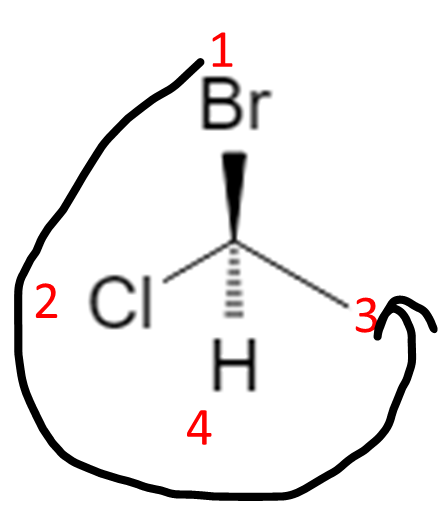
- Trace the path 1 → 2 → 3:
- Clockwise = R (think “right turn”).
- Counterclockwise = S.
- If lowest priority (4) points toward you, assign as drawn, then invert the result.
4.1 Case 1 – Lowest priority is on a dash (ideal)
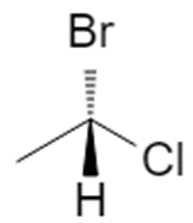
Using 1‑bromo‑1‑chloroethane with H (priority 4) on a dashed bond:
- Priorities: Br (1), Cl (2), CH₃ (3), H (4).
- With H pointing back, the path 1 → 2 → 3 is clockwise → (R).
All these views show the same R configuration because H is behind the plane in each:
4.2 Case 2 – Lowest priority is pointing toward you
If H (priority 4) is on a solid wedge (toward you):
Now you cannot read the trace directly. Conceptually flip the molecule so that 4 goes to the back:
In that correct orientation, if 1 → 2 → 3 is counterclockwise, the configuration is S:
Shortcut many instructors use:
- If 4 is toward you, assign as drawn and then invert R ↔ S.
5. Wedge‑and‑Dash Notation Recap
Wedge‑and‑dash notation encodes 3D shape in a 2D drawing:
- Solid wedge: bond coming out of the page toward you
- Dashed wedge: bond going behind the page
- Straight line: bond lying in the plane of the page
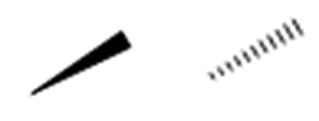
When you’re assigning configuration from any wedge‑and‑dash drawing:
- Put the lowest priority group on a dash (in your mental view).
- Then apply the 1 → 2 → 3 rule to get R or S.
If the drawing doesn’t have 4 on a dash, apply a rotation in your head or use the “assign‑then‑invert” shortcut.
6. Worked Example Summary: 1‑bromo‑1‑chloroethane
- Draw the chiral center with wedges/dashes.
- Rank substituents: Br (1) > Cl (2) > CH₃ (3) > H (4).
- Reorient so H is pointing away.
- Trace 1 → 2 → 3:
- Clockwise → (R)-1-bromo-1-chloroethane
- Counterclockwise → (S)-1-bromo-1-chloroethane
The two are enantiomers: non‑superimposable mirror images with identical properties in achiral media but opposite optical rotations and often different responses in chiral environments (like enzymes or receptors).
7. Key Takeaways
- A chiral center is typically an sp³ carbon attached to four different groups.
- Enantiomers are nonsuperimposable mirror images; they have opposite R/S configurations at each stereocenter.
- Use CIP rules to rank substituents (atomic number, then first point of difference, multiple bonds, isotopes).
- Put the lowest priority group behind, then read 1 → 2 → 3: clockwise = R, counterclockwise = S.
- If 4 is pointing toward you, assign then invert.
- Mastering R/S assignments is essential for interpreting mechanisms, predicting stereochemical outcomes, and understanding how molecules interact in chiral biological systems.