Acid-catalyzed and base-catalyzed reactions
Carbonyl compounds are organic compounds that contain a carbonyl functional group, which consists of a carbon atom double-bonded to an oxygen atom. The carbonyl group is a highly polar group that is involved in many reactions. In this lesson, we will discuss the acid-catalyzed and base-catalyzed reactions of carbonyl compounds.
Acid-Catalyzed Reactions
Acid-catalyzed reactions of carbonyl compounds include hydrolysis, hydration, and formation of acetals and ketals. The mechanism for acid-catalyzed reactions involves the protonation of the carbonyl oxygen, which makes the carbonyl carbon more electrophilic and susceptible to nucleophilic attack. The acid is used to facilitate the protonation of the carbonyl oxygen.
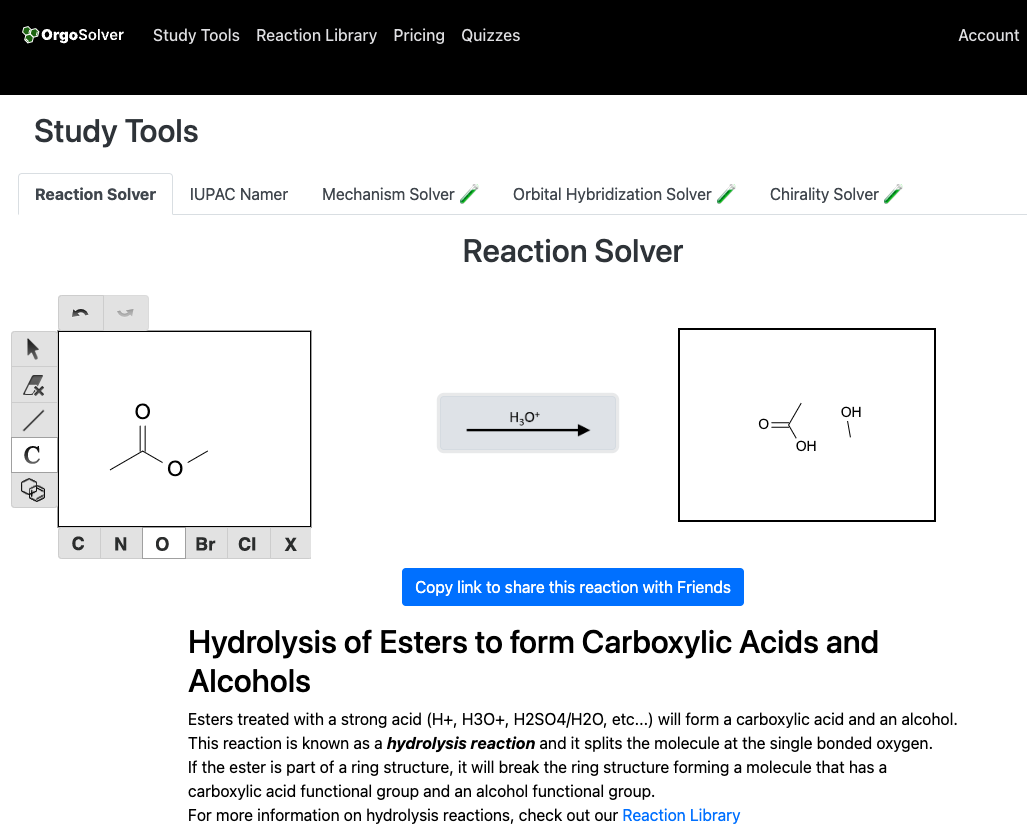
Hydrolysis
Hydrolysis of a carbonyl compound involves the cleavage of the carbonyl group by water, resulting in the formation of a carboxylic acid and an alcohol. For example, the hydrolysis of an ester in the presence of an acid catalyst produces a carboxylic acid and an alcohol.
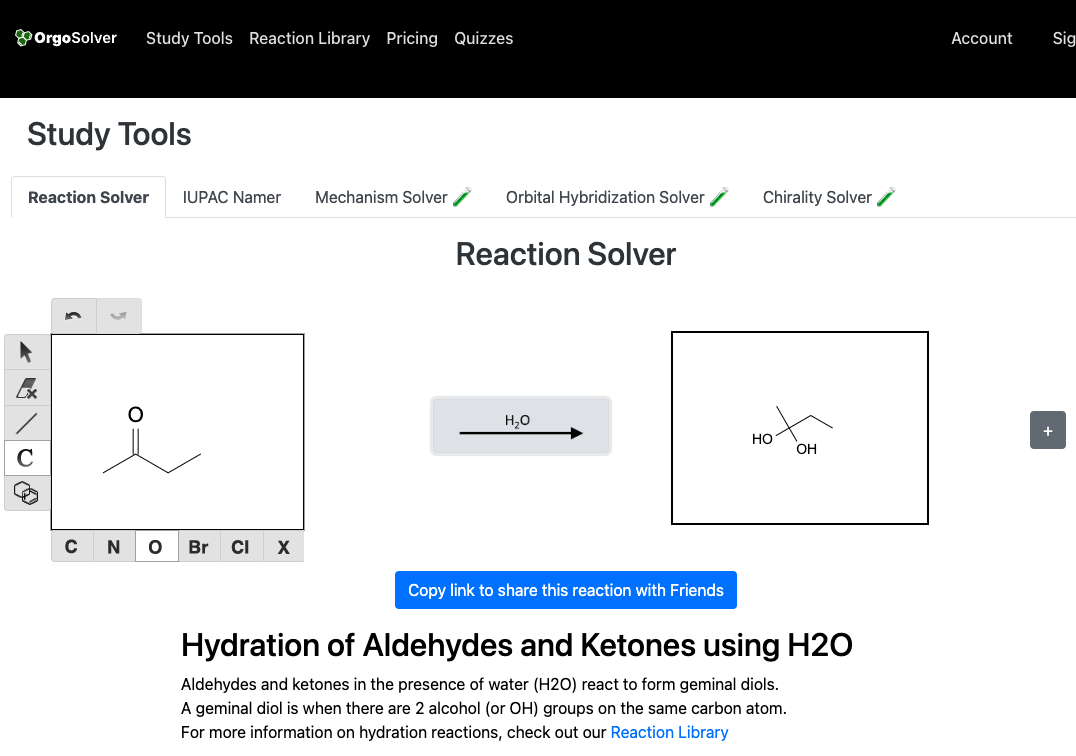
Hydration
Hydration of a carbonyl compound involves the addition of water across the carbonyl group, resulting in the formation of a geminal diol. For example, the hydration of an aldehyde in the presence of an acid catalyst produces a geminal diol.
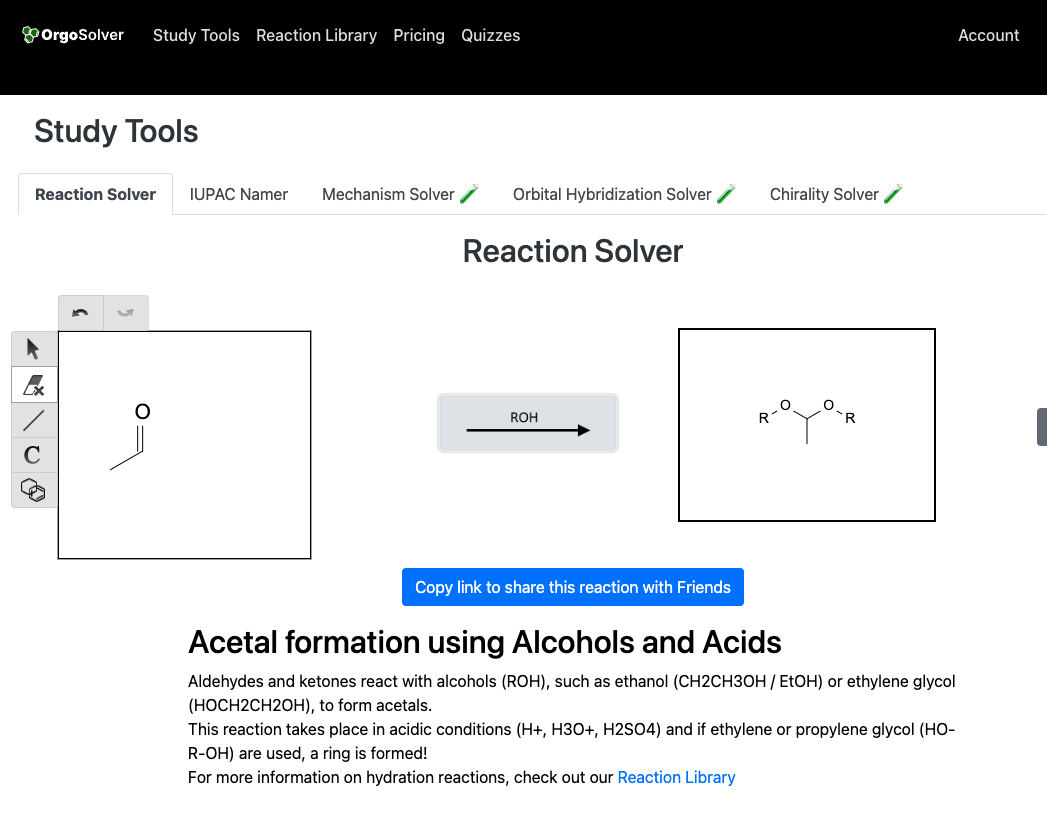
Acetal Formation
Acetal formation involves the reaction of a carbonyl compound with an alcohol to produce an acetal. For example, the reaction of an aldehyde with methanol in the presence of an acid catalyst produces a methyl acetal.
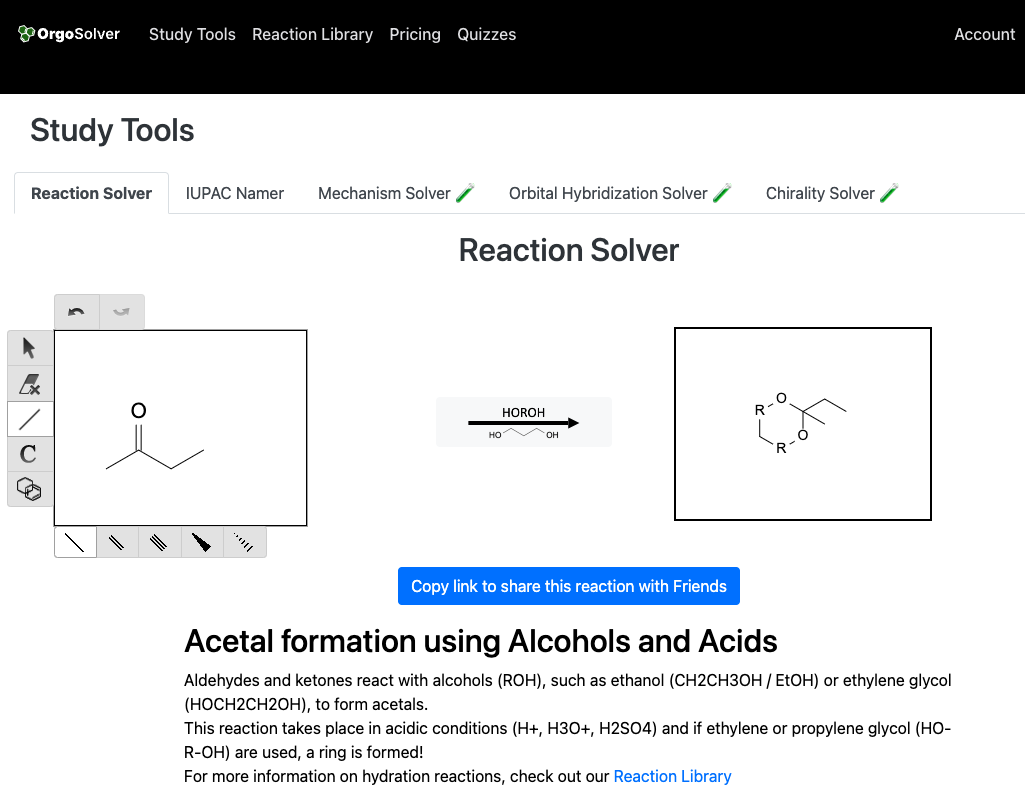
Ketal Formation
Ketal formation involves the reaction of a carbonyl compound with a diol to produce a ketal. For example, the reaction of a ketone with ethylene glycol in the presence of an acid catalyst produces an ethylene glycol ketal.
Base-Catalyzed Reactions
Base-catalyzed reactions of carbonyl compounds include aldol condensation, Michael addition, and Claisen condensation. The mechanism for base-catalyzed reactions involves the deprotonation of the α-carbon adjacent to the carbonyl group, which makes it more nucleophilic and susceptible to attack by an electrophile. The base is used to facilitate the deprotonation of the α-carbon.
For a complete list of carbonyl reactions used in Organic Chemistry 1, check out our Reaction Library!
Visit our Reaction Solver to draw any molecule, select your reagent, and get an answer!
Summary
Carbonyl compounds undergo many acid-catalyzed and base-catalyzed reactions, which can be used to synthesize a wide range of organic compounds. Understanding the mechanisms of these reactions is important for predicting the products of the reactions and designing synthetic routes to desired organic compounds.
Test Your Knowledge:
What is the difference between acid-catalyzed and base-catalyzed reactions of carbonyl compounds?
What is the difference between the mechanism of base-catalyzed aldol condensation and base-catalyzed Michael addition?