Carbonyl Compounds - Nomenclature and Properties
Carbonyl compounds are a class of organic compounds that contain the carbonyl functional group, C=O. This group consists of a carbon atom double-bonded to an oxygen atom, and it imparts characteristic chemical and physical properties to the molecule.
Nomenclature:
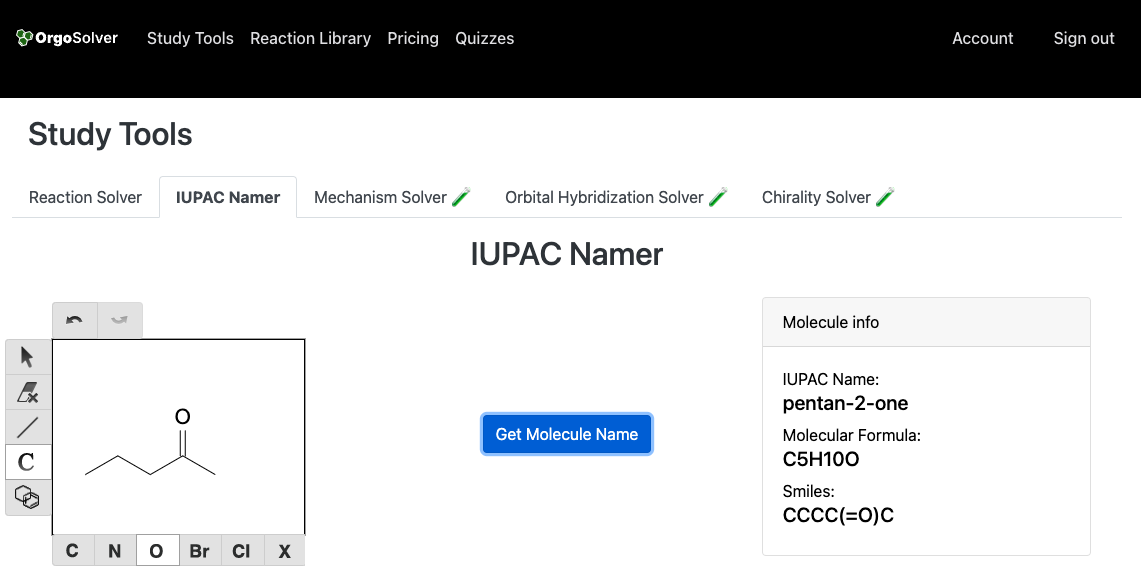
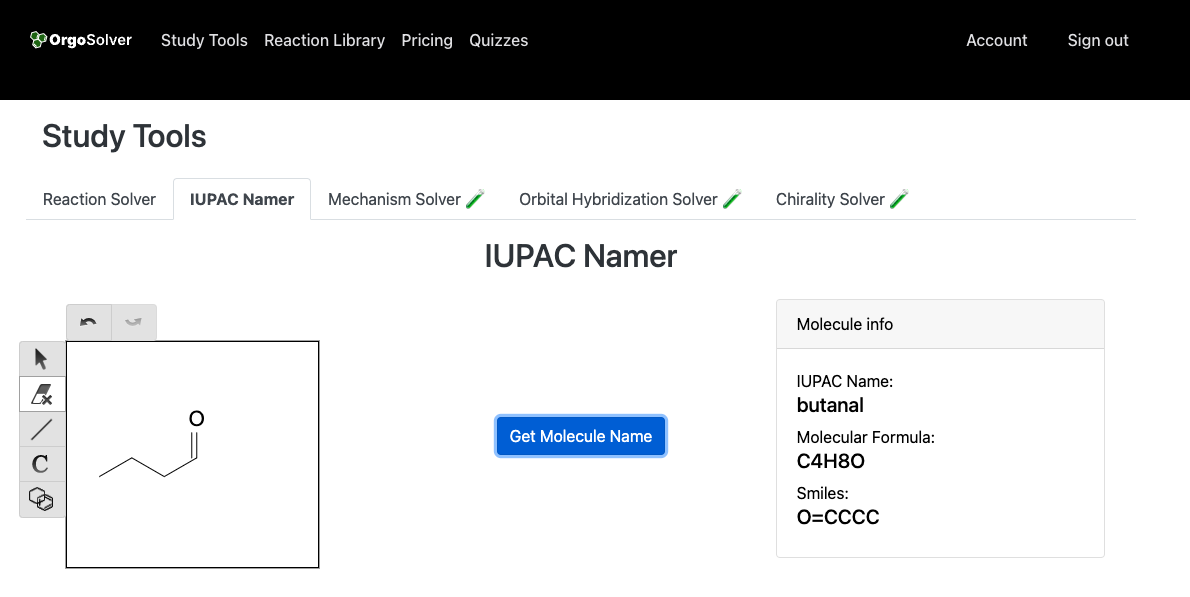
Carbonyl compounds are named by replacing the "e" at the end of the alkane name with "one". In the case of ketones, the parent chain is the longest continuous carbon chain that includes the carbonyl group. For aldehydes, the parent chain is the longest continuous carbon chain that includes the carbonyl group, and the suffix "-al" is added to the end of the alkane name. The carbonyl carbon is always given the lowest possible number.
Pentan-2-one
Butanal
Properties:
The carbonyl group is highly polar due to the difference in electronegativity between carbon and oxygen, and it gives rise to several characteristic properties. For example, carbonyl compounds have higher boiling points than similar-sized hydrocarbons due to the presence of dipole-dipole interactions. Additionally, carbonyl compounds are highly reactive due to the polarity of the carbonyl group, and they can undergo a variety of reactions.
Two real world examples of carbonyl compounds are acetone and propanal. Acetone is a ketone with the formula (CH3)2CO, and it is a common solvent and cleaning agent. Propanal is an aldehyde with the formula CH3CH2CHO, and it is used as a starting material for the synthesis of many other compounds.
Check out our Organic Compound Namer to draw any molecule and get the IUPAC name!
Summary
Carbonyl compounds are a class of organic compounds that contain the carbonyl functional group, C=O. They are named by replacing the "e" at the end of the alkane name with "one" for ketones, and "-al" for aldehydes. The carbonyl group is highly polar and gives rise to several characteristic properties, including higher boiling points and reactivity. Two examples of carbonyl compounds are acetone and propanal.
Test Your Knowledge:
What is the parent chain for an aldehyde?
Why do carbonyl compounds have higher boiling points than similar-sized hydrocarbons?