Aromatic Reactions: Halogenation (Br₂/AlBr₃, Cl₂/AlCl₃)
Aromatic Reactions: Halogenation using Br₂/AlBr₃ (or FeBr₃) and Cl₂/AlCl₃ (or FeCl₃)
Lewis-acid-assisted bromination and chlorination are classic electrophilic aromatic substitution (EAS) reactions. AlBr₃ or AlCl₃—and their ferric counterparts FeBr₃ and FeCl₃—polarize X₂ to an electrophile (“X⁺”), which the aromatic π system attacks to form the σ-complex (Wheland intermediate). Deprotonation restores aromaticity, generating Ar–X while regenerating the Lewis acid. Orientation follows the global EDG/EWG rule set: EDG → ortho/para, EWG → meta; halogens themselves are the canonical o/p-directing deactivators. Chlorination is more aggressive than bromination, so temperature and stoichiometry control are critical to avoid polyhalogenation. Having both Al- and Fe-based Lewis acids available makes it easier to match catalyst compatibility with sensitive substrates (e.g., FeX₃ when AlX₃ complexes too strongly with an amide or directing group).
Key Emphasis (Teaching Pivots)
- Electrophile identity: Br₂/AlBr₃, Br₂/FeBr₃, Cl₂/AlCl₃, or Cl₂/FeCl₃ produces an X⁺-like electrophile (halenium) paired with AlX₄⁻/FeX₄⁻. Always depict Lewis-acid activation before the ring attack.
- Canonical EAS pattern: π-attack → σ-complex (Wheland intermediate with benzylic H explicit) → deprotonation → aromaticity restored.
- Directing control: Use the global EDG/EWG map. Remember the “halogen exception”: halogens are o/p-directing but deactivating overall.
- Selectivity management: Chlorination is more reactive (higher risk of di-/trichlorination). Run cold, meter X₂, and quench promptly.
- Exam trap: Do not confuse ring halogenation with benzylic/allylic radical halogenation (Br₂/hν, NBS). Those are separate mechanisms.
Quick Summary
- Reagents/conditions: Br₂/AlBr₃ or Br₂/FeBr₃; Cl₂/AlCl₃ or Cl₂/FeCl₃ in dry solvent (CH₂Cl₂, CS₂, CCl₄) at 0–25 °C. Keep moisture out to maintain Lewis acidity.
- Electrophile: X⁺ generated by coordination of X₂ to AlX₃ or FeX₃; AlX₄⁻/FeX₄⁻ acts as the conjugate base.
- Outcome: Ar–H → Ar–X (X = Br or Cl). The newly installed halogen is o/p-directing yet deactivating, regardless of whether Al or Fe delivered the electrophile.
- Orientation: EDG/activators → ortho/para (para favored when ortho is blocked). EWG/deactivators → meta. Halogens already on the ring remain o/p-directing.
- Turnover: Deprotonation produces HX; AlX₃ is regenerated from AlX₄⁻ + HX.
- Avoid radicals: No light or peroxides—EAS here is strictly a closed-shell ionic pathway.
Mechanism — EAS Halogenation (5 Frames; arrows A–F)
Each frame below was generated using benzene as the substrate.
Mechanistic Checklist (Exam Focus)
- Always show Lewis-acid activation of X₂; do not skip directly to ring attack.
- Three canonical EAS frames: electrophile generation → σ-complex → deprotonation/turnover.
- Halogens as substituents are o/p-directing yet deactivating—mention this exception explicitly when explaining regioselectivity.
- Chlorination runs hotter/faster; emphasize temperature control to avoid multiple substitutions.
- Avoid radical arrows or hv/ROOR notation—different mechanism entirely.
Worked Examples
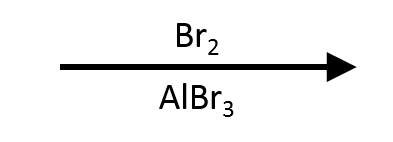
Monobromination proceeds cleanly at 0–25 °C with Br₂/AlBr₃ (or FeBr₃). This panel anchors the mechanism frames and regression tests.
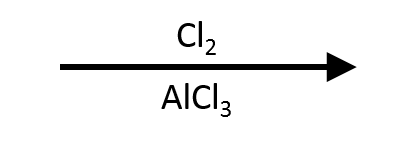
Chlorination is more aggressive; run colder (0–10 °C) and meter Cl₂ slowly to keep mono-chlorination under control.
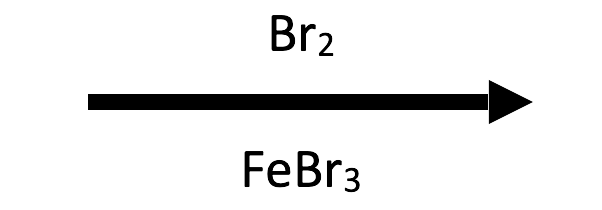
Ferric halides (FeBr₃/FeCl₃) behave identically to the aluminum salts; swap them in when AlX₃ is incompatible with the substrate or when you already have FeX₃ on hand.

The router displays both ortho and para isomers, with para typically favored by sterics when the ortho site is crowded.

Strongly deactivated rings (e.g., –NO₂) react sluggishly and require warmer/longer conditions; meta selectivity dominates.
Scope & Limitations
- Reactive substrates: Benzene, alkylbenzenes, anisoles, and phenols halogenate quickly; keep the run cold to avoid over-halogenation.
- Deactivated rings: –NO₂, –CF₃, –SO₃H, carbonyl clusters react slowly (or fail) unless heated; expect lower yields.
- Lewis-acid sensitivity: Strong donors (–NH₂, –OH) can bind or be protonated; protect (e.g., acetanilide) to retain predictable orientation, or swap from AlX₃ to FeX₃ when aluminum complexes too tightly.
- Polyhalogenation risk: Chlorination is particularly prone to multiple substitutions; limit equivalents, temperature, and reaction time.
- Halogen-on-halogen: Halobenzenes remain o/p-directing but are deactivated, so additional halogenation is slower and typically para-selective.
Practical Tips
- Dry everything: Solvents and glassware must be dry; moisture quenches AlX₃ and derails the reaction.
- Add halogen slowly: Meter Br₂/Cl₂ into a cooled arene/AlX₃ mixture to control the exotherm and maintain mono-halogenation.
- Quench carefully: Destroy excess halogen with Na₂S₂O₃, then neutralize the Lewis acid cautiously (aqueous quench generates HX heat).
- Catalyst swap awareness: Keep both AlX₃ and FeX₃ on hand—switch to FeBr₃/FeCl₃ when Al salts foul by coordination or when an Fe catalyst is already present downstream.
- Block when needed: Combine sulfonation/desulfonation with halogenation to steer o/p access for multi-step syntheses.
- Avoid hv/ROOR: Radical conditions target benzylic/allylic C–H bonds, not the ring.
Exam-Style Summary
Br₂/AlBr₃ or Cl₂/AlCl₃ polarizes X₂ into an electrophile (X⁺). The arene’s π bond attacks to give a σ-complex, AlX₄⁻ removes the benzylic proton, and aromaticity returns with Ar–X formed plus HX (AlX₃ regenerated). Orientation follows EDG/EWG logic; halogens are o/p-directing but deactivating. Chlorination is more vigorous than bromination—keep it cold to avoid polyhalogenation, and never confuse this ring halogenation with benzylic radical reactions.
Interactive Toolbox
- Mechanism Solver — Step through aromatic halogenation (Br₂/Cl₂ with AlX₃ or FeX₃) frame by frame with overlays/arrows and narrated cues.
- Reaction Solver — Predict ortho/meta/para products for substituted arenes under Br₂/Cl₂ + Lewis acid while flagging polyhalogenation risk.
- IUPAC Namer — Practice naming haloarenes (bromobenzene, p-bromotoluene, m-bromonitrobenzene, etc.) generated by this reaction.