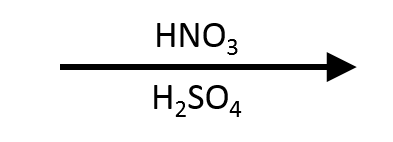
Aromatic Reactions: Nitration with HNO₃/H₂SO₄
Aromatic Reactions: Nitration using HNO₃/H₂SO₄
Mixed acid is simply concentrated nitric acid dissolved in concentrated sulfuric acid. The strong dehydration by H₂SO₄ protonates HNO₃, ejects water, and liberates nitronium ion (NO₂⁺). Every electrophilic aromatic substitution (EAS) text shows the same choreography: the arene π bond attacks NO₂⁺, the σ-complex (Wheland intermediate) captures the positive charge, and HSO₄⁻ (or H₂O) removes the benzylic proton to re-form the aromatic π system. The nitro group that appears is powerfully deactivating and meta-directing, so nitration is both a synthesis tool (gateway to anilines) and a directing-pattern teaching moment.
Key Emphasis (Teaching Pivots)
- Electrophile identity: HNO₃ + H₂SO₄ (“mixed acid”) rapidly generates nitronium ion (NO₂⁺); never draw “HNO₃ attacks the ring.”
- Canonical EAS flow: π-attack → σ-complex (Wheland intermediate with explicit benzylic H) → deprotonation restores aromaticity and regenerates acid.
- Directing logic: EDG/activators give ortho/para, EWG/deactivators give meta, halogens remain the classic o/p-but-deactivating exception. Once –NO₂ is installed it enforces meta logic for subsequent EAS.
- Temperature control: Cool (0–55 °C) runs limit polynitration; hotter or fuming conditions increase [NO₂⁺] and risk dinitration.
- Aniline trap: Free –NH₂ is protonated to –NH₃⁺ under mixed acid, flipping to meta-directing. Protect as acetanilide to retain o/p nitration, then hydrolyze.
- Assets/tests baseline: Default mechanism frames and tests use benzene; router overlays then swap in substituted arenes (toluene, anisole, nitrobenzene, acetanilide) for directing scenarios.
Quick Summary
- Reagents/conditions: Concentrated HNO₃ in concentrated H₂SO₄ (“mixed acid”), typically 0–55 °C; forcing temperatures or fuming acid increase NO₂⁺ concentration.
- Electrophile: NO₂⁺ generated via H₂SO₄-protonated nitric acid followed by water loss (A → B in the mechanism art).
- Outcome: Ar–H → Ar–NO₂; the nitro group is strongly deactivating, meta-directing, and readily reduced to –NH₂ downstream.
- Mechanism: Hydration-free nitronium formation → π attack to give σ-complex → HSO₄⁻ (or H₂O) removes the benzylic proton → aromaticity restored.
- Directing control: EDG (–OR/–NR₂/alkyl) favor para > ortho when sterics block ortho; EWG (–NO₂, –SO₃H, –C=O families) enforce meta; halogens = o/p but slow.
- Process control: Keep mixtures cold, add arene to acid (not vice versa), and quench quickly to avoid poly-nitration, oxidation, or dark tars.
Mechanism — Mixed-Acid Nitration (4 Frames; arrows A–F)
Each frame uses benzene as the baseline substrate.
Mechanistic Checklist (Exam Focus)
- Always show nitronium (NO₂⁺) generation before the ring attack—no “HNO₃ arrow directly into benzene.”
- Rate-determining step = π-attack forming the σ-complex.
- The σ-complex should include the benzylic H and the adjacent carbocation so the deprotonation arrows make sense.
- Aniline is protonated (–NH₃⁺) under mixed acid → meta; acetanilide stays o/p. State this explicitly when analyzing directing conflicts.
- –NO₂ is strongly deactivating and meta-directing; halogens are the only o/p-but-deactivating exception.
- Temperature and acid strength modulate poly-nitration—draw attention to conditions when explaining selectivity.
Worked Examples
All reagent badges reuse the exact PNG that appears in the Reagent Search grid. Each figure shows Reactant → Reagent button → Product (single-stage process).
Mixed acid (40–55 °C) converts benzene cleanly into nitrobenzene. This example anchors the mechanism frames, the regression tests, and the Reaction Solver baseline.

The router favors para > ortho whenever sterics allow, so the asset shows both products joined by “+”. Keeping para dominant but still displaying ortho visually reinforces the EDG rule set.

Free aniline is protonated to –NH₃⁺ (meta-directing), whereas acetanilide stays neutral and directs ortho/para. Showing both panels next to the same reagent emphasizes the protecting-group logic students must state on exams.

Under forcing temperatures (>80 °C) nitrobenzene can be driven to m-dinitrobenzene. The figure doubles as a cautionary tale about polynitration when the run gets too hot.
Scope & Limitations
- Smooth substrates: Benzene, alkylbenzenes, anisoles, phenols (run cold), and mildly activated rings nitration readily.
- Challenging substrates: Strongly deactivated rings (–CF₃, –NO₂, –SO₃H, carbonyl clusters) require hotter or more concentrated acid; expect slower rates and lower yields.
- Functional-group sensitivity: Acid-sensitive protecting groups or oxidizable side chains may not survive mixed acid. Consider using sulfonation/desulfonation blocking strategies or milder nitrating mixtures when possible.
- Polynitration risk: Elevated temperature, long dwell times, or highly activated rings invite di/trinitration. Keep the bath cold and quench promptly.
- Metal coordination: Strong donor groups (e.g., thiols) can bind or quench nitronium; mask them before nitration.
Practical Tips
- Charge nitric acid into sulfuric acid (never reverse) while cooling to keep the exotherm under control.
- Use glass or PTFE tools—mixed acid attacks many metals and most elastomers.
- Add the arene slowly into the cooled mixed acid, monitor temperature, then quench by pouring onto crushed ice and neutralizing carefully.
- Limit exposure time once nitration is complete; prolonged hot residence invites oxidation, tar, or over-nitration.
- Plan downstream: Nitro groups reduce smoothly (Fe/HCl, Sn/HCl, catalytic hydrogenation), so nitration is a standard entry point to anilines.
Exam-Style Summary
HNO₃/H₂SO₄ (mixed acid) generates nitronium ion (NO₂⁺). The arene’s π bond attacks NO₂⁺ to form a σ-complex; HSO₄⁻ (or H₂O) deprotonates to restore aromaticity and deliver Ar–NO₂. The nitro group is strongly deactivating and meta-directing—protect amines (acetanilide) when you need o/p nitration, and keep temperatures low to avoid polynitration.
Exam cues / pitfalls
- Forgetting to show NO₂⁺ formation or drawing “HNO₃ attacks benzene.”
- Neglecting the σ-complex frame, which leaves nowhere for the proton-abstraction arrow to land.
- Claiming anilines direct o/p under mixed acid without protecting the amine—state the protonation issue.
- Ignoring temperature/time when asked how to avoid di- or trinitration of activated rings.
Interactive Toolbox
- Mechanism Solver — Step through the HNO₃/H₂SO₄ nitration mechanism with narrated explanations, highlighting nitronium formation and σ-complex frames.
- Reaction Solver — Predict ortho/meta/para outcomes for any arene under mixed acid and surface warnings when conditions risk polynitration.
- IUPAC Namer — Practice naming nitroaromatic products (e.g., 4-nitrotoluene, m-dinitrobenzene) without exposing SMILES in the learner copy.