Alkene Reactions: Addition of Alcohols to Alkenes using Acids
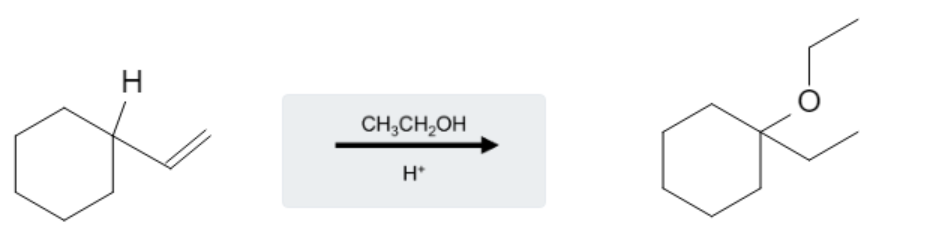
Using an aqueous acid, such as H2SO4 or H+, alongside alcohols, such as CH3OH and CH3CH2OH, as reagents in reaction with alkenes will result in the formation of an alcohol:
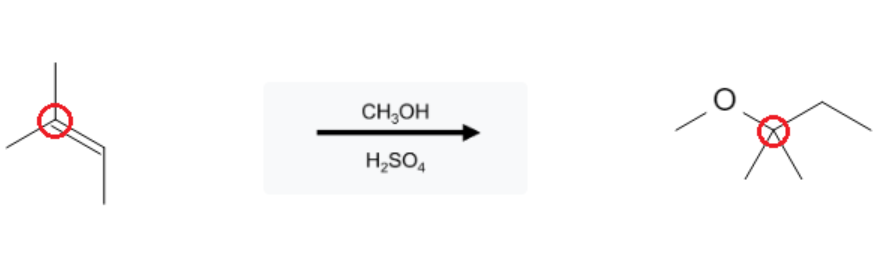
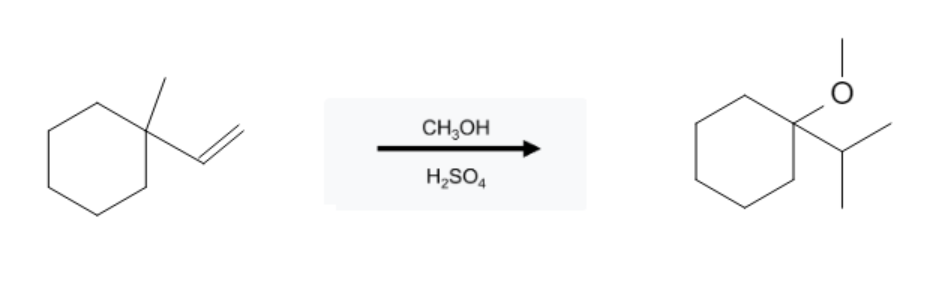
The reaction takes place on the most substituted carbon, following Markovnikoff selectivity. Additionally, hydride and/or methyl rearrangement can occur, causing the alcohol group to be added to a more highly substituted carbon adjacent to the alkene bond:
Hydride Shift
Methyl Shift
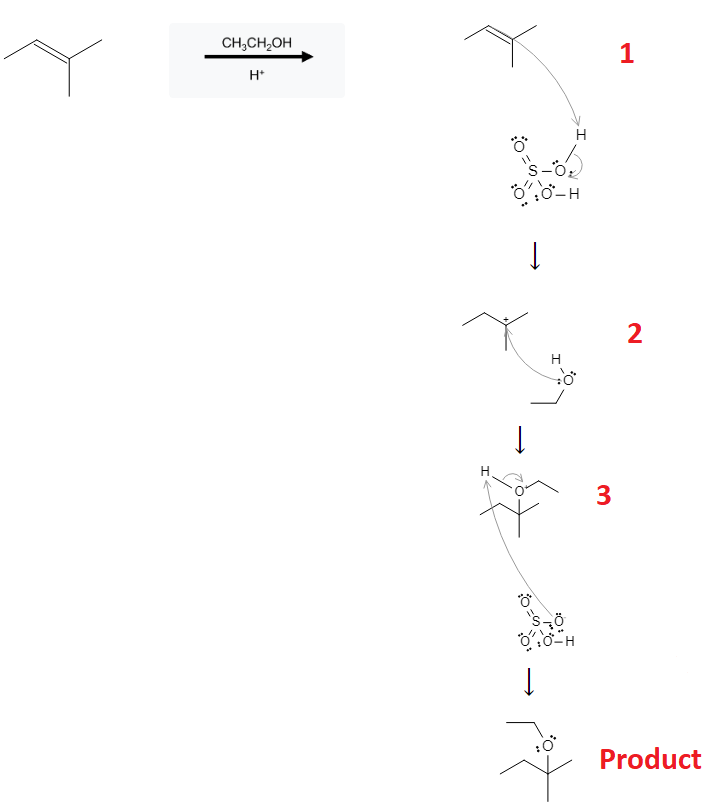
The reaction mechanism is depicted below:
In the first step, the alkene is protonated by the acid, removing the double bond and causing 1 carbon to carry a positive charge. In the second step, methanol (CH3OH) or ethanol (CH3CH2OH) arrives at the reaction site, attaching to the molecule in a nucleophilic attack. The third reaction step involves the deprotonated acid (HSO4-) molecule using its free electrons to deprotonate the oxygen, resulting in a regeneration of the acid as well as an alcohol functional group being added to the original molecule.
The acids commonly used for this reaction (but not exclusive) are: H2SO4, H3O+, and H+.