Aromatic Reactions: SNAr — Nucleophilic Aromatic Substitution via Meisenheimer Complex
A Meisenheimer mechanism is the most common SNAr (nucleophilic aromatic substitution) pathway taught in Orgo I/II. It applies when an aromatic ring has:
- a leaving group (usually an aryl halide), and
- one or more strong electron-withdrawing groups (EWGs) positioned to stabilize an anionic intermediate.
The key intermediate is the Meisenheimer complex (a resonance-stabilized anionic σ-complex). The reaction is addition first (aromaticity is lost), then elimination (aromaticity is restored).
Quick Summary
What you need for Meisenheimer (SNAr)
A typical SNAr substrate requires:
- A strong nucleophile (often O−, amines, or CN−)
- A leaving group on the ring (often F, Cl, Br)
- At least one anion-stabilizing EWG ortho and/or para to the leaving group (commonly NO&sub2;; also CN, carbonyls, sulfones)
Why ortho/para matters
The anionic σ-complex is stabilized only if the negative charge can be delocalized into the EWG by resonance. If the EWG is meta, that stabilization pattern is not available, and the SNAr addition step becomes too unfavorable.
The counterintuitive leaving group trend
In many SNAr reactions, aryl fluoride reacts fastest (often: F ≫ Cl ≈ Br ≫ I). This is not because F− is "the best leaving group" in the elimination step; it's because the rate depends mainly on the addition step, and a strongly electron-withdrawing C–F bond helps the ring accept electron density.
Predicting the Major Product
Use this checklist:
-
Is there a strong EWG ortho/para to X?
- Yes → SNAr/Meisenheimer is plausible
- No → SNAr is usually not the right answer under mild conditions (see benzyne trap)
-
Which carbon is attacked?
- For Meisenheimer SNAr, attack occurs at the ipso carbon bearing the leaving group
-
Which leaving group departs?
- The leaving group is expelled when aromaticity is restored
-
If the nucleophile is neutral (amines, alcohols):
- Account for an extra deprotonation step
Mechanism (4 Steps)
Worked Examples
NaOMe (Methoxide)
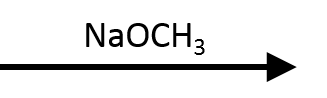
NaOH (Hydroxide)
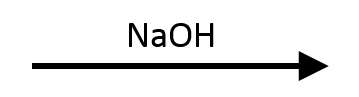
NaOEt (Ethoxide)
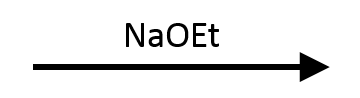
NaSH (Thiolate)
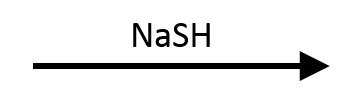
NH&sub3; (Ammonia)

KCN (Cyanide)
Exam Traps and Edge Cases
1. No EWG = No Meisenheimer SNAr
Unactivated chlorobenzene/bromobenzene generally do not undergo Meisenheimer SNAr under typical conditions. If a problem shows very strong base and harsh conditions, the mechanism may instead be benzyne (elimination–addition), often giving mixtures/regioisomers.
2. Meta EWG Does Not Activate
A meta nitro/cyano/carbonyl group cannot stabilize the σ-complex by the key resonance pathway, so Meisenheimer SNAr is typically not the intended answer.
3. Leaving Group Order is "Weird"
Aryl fluoride is often the most reactive substrate (F ≫ Cl ≈ Br ≫ I). On exams, this is a diagnostic clue for a Meisenheimer addition–elimination pathway.
4. Don't Confuse with EAS σ-Complex
EAS also forms a σ-complex, but it's a carbocation intermediate (arenium ion); the Meisenheimer complex is typically anionic (or zwitterionic before deprotonation).
5. Benzyne Alternative
If the substrate lacks activating EWGs but the conditions include strong base (like NaNH&sub2;) and heat, consider the benzyne mechanism instead of SNAr.
Product Prediction Checklist
- Identify an aryl leaving group (usually halide)
- Check for strong EWG ortho/para to that leaving group
- Choose Meisenheimer (SNAr) if activated; otherwise consider "no reaction" or "benzyne"
- Use an added deprotonation step if Nu is neutral (amines/alcohols)
- Verify that the final step restores aromaticity by expelling X−
FAQ
Q: Why does aryl fluoride react faster than aryl iodide in SNAr?
The rate-determining step is nucleophile addition, not leaving group departure. The highly electronegative C–F bond helps stabilize the incoming negative charge, making addition faster. This is the opposite of SN2 on alkyl halides.
Q: Can SNAr happen without any EWG?
Generally no. Without EWG stabilization of the Meisenheimer complex, the activation energy is too high. Under very forcing conditions, the benzyne mechanism may operate instead.
Q: What's the difference between SNAr and EAS?
EAS involves electrophile attack on an electron-rich ring, forming a cationic σ-complex (arenium ion). SNAr involves nucleophile attack on an electron-poor ring, forming an anionic σ-complex (Meisenheimer complex). They are essentially opposites.
Interactive Toolbox
- Mechanism Solver — draw a reaction and see the full arrow-pushing mechanism.
- Reaction Solver — predict products from substrates and reagents.
- IUPAC Namer — get systematic names for organic molecules.