IUPAC Naming Masterclass
IUPAC Naming Masterclass
Use IUPAC Namer to follow along to this lesson and practice yourself!
1. Parent-chain selection
The first step in IUPAC naming is to identify the parent chain—the longest continuous chain of carbon atoms that includes the highest-priority functional group.
- Find the longest continuous carbon chain containing the principal functional group.
- If two chains tie, choose the one with more substituents or multiple bonds.
- Number the chain so the principal functional group gets the lowest possible locant.
By choosing the true longest chain first, you guarantee that all later steps (functional-group numbering, substituent naming) fall into place correctly.
See the 3-methylhexane below:
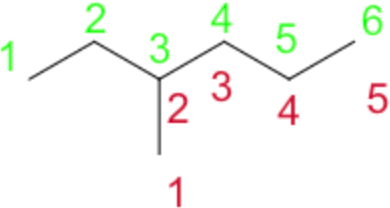
By starting at the green carbon 1, six carbon atoms are able to be linked in a continous chain. If you had selected the carbon at position 3, the longest continous carbon chain possible would have been five carbon atoms. As such, you would consider the main chain to be the 6 carbons in a row, and the functional group on carbon 3.
2. Functional-group priority table (highest → lowest)
The table below summarizes the most common functional groups encountered in organic chemistryL
| Priority | Suffix / Ending | Example IUPAC name | Image |
|---|---|---|---|
| 1 | -oic acid (carboxylic acid) | ethanoic acid | 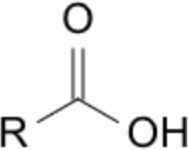 |
| 2 | -oyl chloride (acyl chloride) | propanoyl chloride | 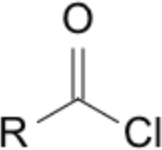 |
| 3 | -amide | butanamide | 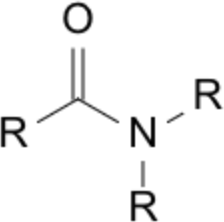 |
| 4 | -nitrile | ethanenitrile |  |
| 5 | -al (aldehyde) | propanal | 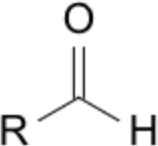 |
| 6 | -one (ketone) | butan-2-one | 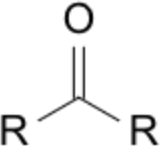 |
| 7 | -ol (alcohol) | butan-1-ol | 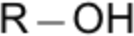 |
| 8 | -amine | ethan-1-amine | 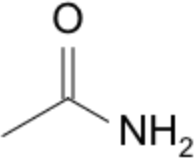 |
| 9 | -ene / -yne (double/triple bonds) | but-1-ene |  |
| 10 | -ane (alkane) | propane |  |
When the principal group isn’t highest priority (e.g., naming an alcohol in presence of an aldehyde), it is treated as a prefix (hydroxy-, oxo-, cyano-, etc.).
3. Numbering & locant rules
- Start numbering from the end that gives lowest numbers to the principal group.
- Double and triple bonds should receive the lowest locant set possible.
- Indicate multiple identical substituents with prefixes di-, tri-, tetra- but do not alphabetise prefixes (alphabetise the root name).
Lets examine 3-methylpent-2ene as an example:
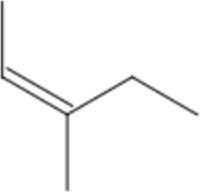 ```
3-methylpent-2-ene
```
```
3-methylpent-2-ene
```
- First count the parent chain length = 5 (pent- prefix)
- Next, identify any alkene/alkyne bonds. There is a Double bond at C-2 (-2-ene)
- Finally, locate all substituents that are not part of the parent chain. There is a methyl substituent at C-3
Done!
4. Substituent Naming & Alphabetical order
After you’ve picked your parent chain, name each substituent and list them in alphabetical order.
- Ignore multiplying prefixes (di-, tri-, etc.) for ordering
- Include structural prefixes (iso-, neo-, cyclo-) in the alphabetization
| Substituent | Prefix | Alphabetical key | Example name | Image |
|---|---|---|---|---|
| –NH₂ | amino- | a | 2-amino-4-methylhexane |  |
| cyclopropyl– | cyclopropyl- | c | 1-cyclopropyl-3-methylcyclohexane | 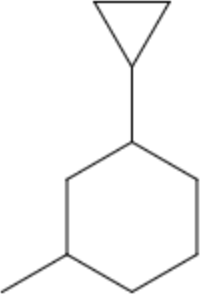 |
| –OCH₃ | methoxy- | m | 3-methoxy-2-methylpentane | 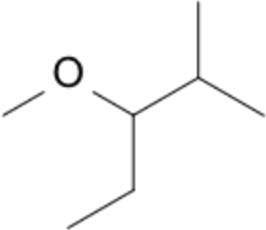 |
| –CH₃ | methyl- | m | 2-methylbutane | 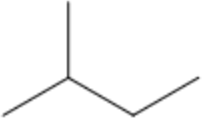 |
| CH₃CH₂– | ethyl- | e | 1-ethyl-3-methylcyclopentane | 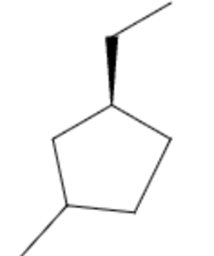 |
| CH₃CH₂CH₂– | propyl- | p | 2-methyl-4-propylheptane | 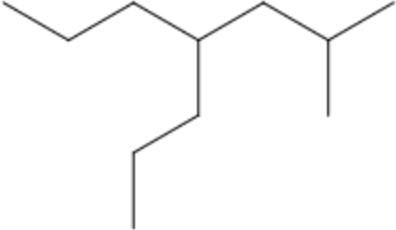 |
| (CH₃)₃C– | tert-butyl- | b | 4-tert-butylheptane | 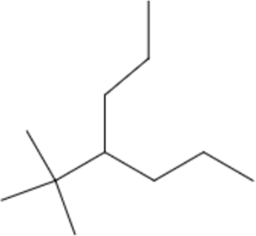 |
Alphabetical example:
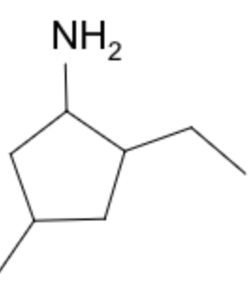
For 2-ethyl-4-methylcyclopentan-1-amine , order is ethyl (e) → methyl (m) → amine (a) Note that amine is last because it is part of the principal group since it is of high importance than ethyl or methyl groups, and therefore assigned as a suffix instead of a prefix
Alphabetical order ignores di-, tri-, sec-, tert- and focuses on the molecule "underneath" (such as tert-butlyheptane).
5. Multiple bonds & ring systems
5.1 Alkenes & Alkynes
Let's take a look at but-1-ene and pent-2-yne:
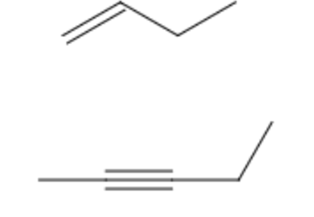
- Lower of the two sp² carbons determines the locant: but-1-ene, pent-2-yne.
- For enynes (double + triple), “-en-yne” with lowest locant set: hept-2-en-5-yne.
5.2 Cyclic compounds
For cyclic compounds, add the prefix cyclo- to the ring size: cyclohexane.

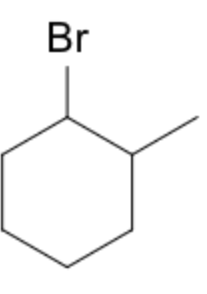
If substituents are added to the molecule, the numbering follows lowest-set rule: 1-bromo-2-methylcyclohexane.
6. Basic stereochemical descriptors
| Case | Descriptor | Example | Image |
|---|---|---|---|
| double bond geometry | E/Z | E-but-2-ene | 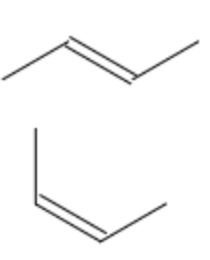 |
| chiral centre | R/S | (S)-2-chlorobutane | 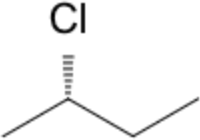 |
| cis/trans in simple rings | cis / trans | cis-1,2-dimethylcyclopropane | 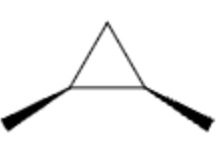 |
To determine if an alkene is (E) or (Z), the most common trick is to assess the substituents and figure out which are the two biggest substituents. Next, if they are opposite, you assign them as (E), if they are on the same side, you assign them as (Z). The trick to remember here is the follow phrase: "ze zame zide" meant to represent "the same side", and this will be a (Z) molecule.
- Write descriptors in parentheses before the name, separated by commas if multiple.
- For multiple chiral centers:
(2R,3S)-2,3-dihydroxybutanedioic acid.
To practice, try our IUPAC Namer and draw any molecule you can imagine!