Reactivity and Reactions of Aromatic Compounds: Nucleophilic Aromatic Substitution
Aromatic compounds, known for their stability and unique electronic structure, exhibit distinct reactivity patterns compared to other organic compounds. Understanding the reactivity of aromatic compounds is essential in organic chemistry as it enables the prediction and control of their chemical transformations. In this lesson, we will explore the reactivity and various reactions of aromatic compounds, shedding light on important concepts and mechanisms. This lesson will focus on Nucleophilic Aromatic Substitution.
Nucleophilic Aromatic Substitution (NAS)
Nucleophilic aromatic substitution (NAS) is a type of reaction that involves the substitution of a nucleophile onto an aromatic ring. Unlike electrophilic aromatic substitution (EAS), which involves the attack of an electrophile, NAS reactions proceed through the attack of a nucleophile on an electron-deficient aromatic system.
The reactivity of aromatic compounds towards NAS reactions is influenced by the electronic and steric effects of substituents on the aromatic ring. Electron-withdrawing groups increase the reactivity of the ring towards nucleophilic attack, while electron-donating groups decrease the reactivity. Steric hindrance around the reaction site can also affect the reaction rate, with bulky substituents often hindering the approach of nucleophiles.
Nucleophilic aromatic substitution reactions can proceed via two different mechanisms: the benzyne mechanism and the addition-elimination mechanism. In the benzyne mechanism, an elimination step generates a highly reactive benzyne intermediate, which then undergoes nucleophilic attack. In the addition-elimination mechanism, the nucleophile adds directly to the aromatic ring, followed by elimination of the leaving group.
Lets take a closer look at the benzyne mechanism:
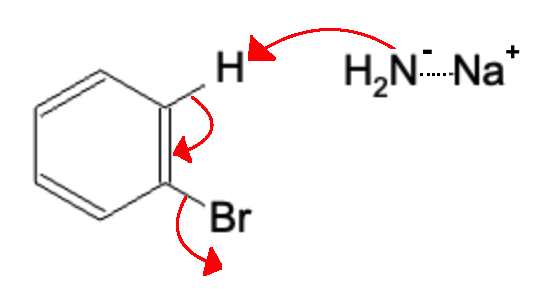
Benzene molecule possesses a good leaving group, such as Br
First, a strong base, such as NaNH2, eliminates a hydrogen atom adjacent to the leaving group, causing the group to leave and forming the benzyne intermediate:
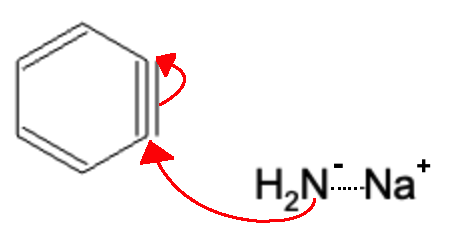
Next, another NaNH2 group attacks the benzyne, forcing the electrons to break the triple bond and move to the adjacent carbon atom.
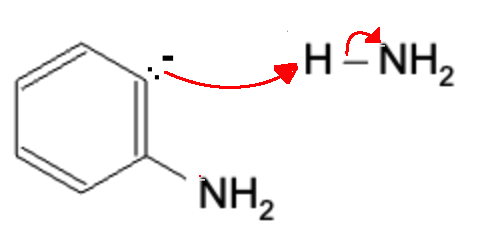
The previously formed NH3 molecule is attacked by the lone pair, thereby regenerating the NH2- molecule.
This completes the reaction with the final product below:
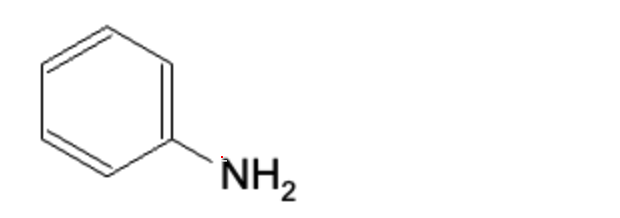
Addition Elimination Mechanism
This mechanism will be more familiar to Sn1 type reactions where we will have 2 steps for the substitution.
Nucleophilic attack on leaving group carbon, causing double bond to become lone pair
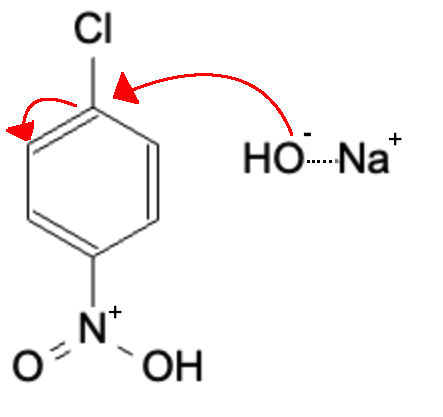
Lone Pair reforms double bond, kicking off the leaving group (Cl)
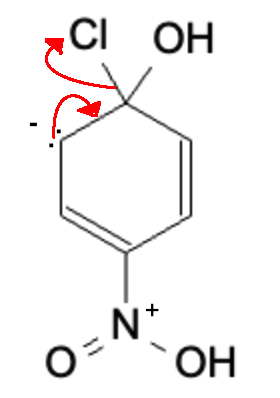
Final Product
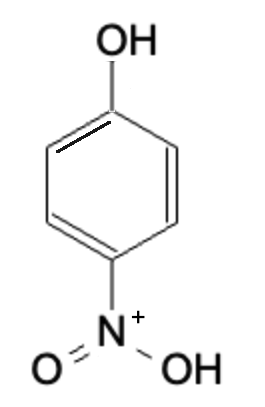
Summary
Nucleophilic aromatic substitution is a versatile reaction that allows for the introduction of functional groups onto aromatic rings. Unlike electrophilic aromatic substitution, which involves the attack of electrophiles on aromatic systems, NAS reactions can proceed via the benzyne mechanism, forming highly reactive benzyne intermediates or through the addition-elimination mechanism.
In NAS reactions, a strong base is used to deprotonate the ortho or para position of the aromatic ring, generating a nucleophilic carbanion. This carbanion then attacks the benzyne intermediate, leading to the substitution of the leaving group. Electron-rich nucleophiles, such as amines, thiols, and phosphines, can participate in NAS reactions.
Coupling reagents, such as copper salts or palladium catalysts, can be employed to facilitate the coupling of aryl halides or aryl boronic acids with nucleophiles in the presence of the benzyne intermediate. Leaving groups, such as halides or sulfonates, are important in initiating the formation of the benzyne intermediate.
It is essential to consider the specific reaction conditions, choice of reagents, and safety precautions when conducting nucleophilic aromatic substitution reactions.
Overall, understanding the principles of nucleophilic aromatic substitution and its applications in organic synthesis expands the toolkit for modifying aromatic compounds and enables the synthesis of diverse and functionalized aromatic derivatives.
Test Your Knowledge:
What type of reagent is needed to begin a NAS reaction?
A) Strong Acid
B) Strong Electrophile
C) Strong Base
D) Strong Nucleophile
E) C or D
What is the mechanism for a benzyne NAS reaction?