Grignard Reagents, Organolithium Compounds, and Gilman Reagents
In the realm of organometallic compounds, Grignard reagents, organolithium compounds, and Gilman reagents stand as stalwart warriors, wielding their remarkable reactivity and versatility in organic synthesis. These compounds, known for their carbon-metal bonds, have become indispensable tools for chemists worldwide. In this lesson, we will delve into the world of Grignard reagents, organolithium compounds, and Gilman reagents, exploring their preparation, properties, reactions, and transformative impact on organic chemistry.
Preparation and Properties:
Grignard reagents are typically prepared by reacting alkyl or aryl halides with magnesium metal. Organolithium compounds, on the other hand, are formed through the reaction of alkyl or aryl halides with lithium metal or organolithium compounds with themselves. Both classes of compounds are highly reactive and sensitive to air and moisture, requiring rigorous handling under inert conditions.
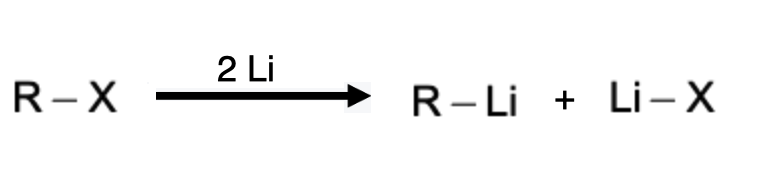
Prepartion of Organolithium Compounds
Organolithium compounds are typically prepared by the direct reaction of alkyl or aryl halides with metallic lithium, often carried out in anhydrous and inert solvents such as ether or tetrahydrofuran. The reaction proceeds through a radical mechanism, wherein the metal-lithium bond undergoes homolytic cleavage, generating an alkyl or aryl radical and a lithium halide salt. The alkyl or aryl radical subsequently reacts with another lithium atom to form the organolithium compound. This process is highly reactive and requires careful control of reaction conditions, including temperature, reaction time, and stoichiometry, to ensure the desired product formation.
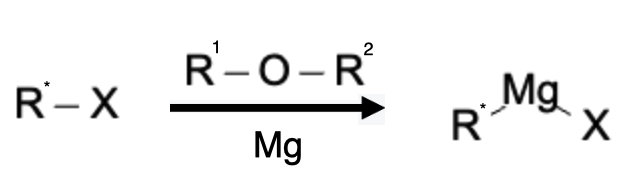
Preparation of Grignard Reagents
Grignard reagents are typically prepared by the reaction of alkyl or aryl halides with magnesium metal in the presence of anhydrous ether or tetrahydrofuran solvents. This process, known as the Grignard reaction, involves the formation of a bond between the carbon atom of the halide and the magnesium atom. The reaction proceeds through a radical mechanism, initiated by the insertion of a metal atom into the carbon-halogen bond, followed by the coordination of the resulting radical with additional magnesium atoms to form the Grignard reagent. It is crucial to carry out the reaction under anhydrous conditions since water can readily react with the highly reactive Grignard reagent. Grignard reagents are versatile nucleophiles and strong bases, enabling the synthesis of a wide range of organic compounds, including alcohols, ketones, carboxylic acids, and more.
Reactivity of Grignard Reagents and Organolithium Compounds:
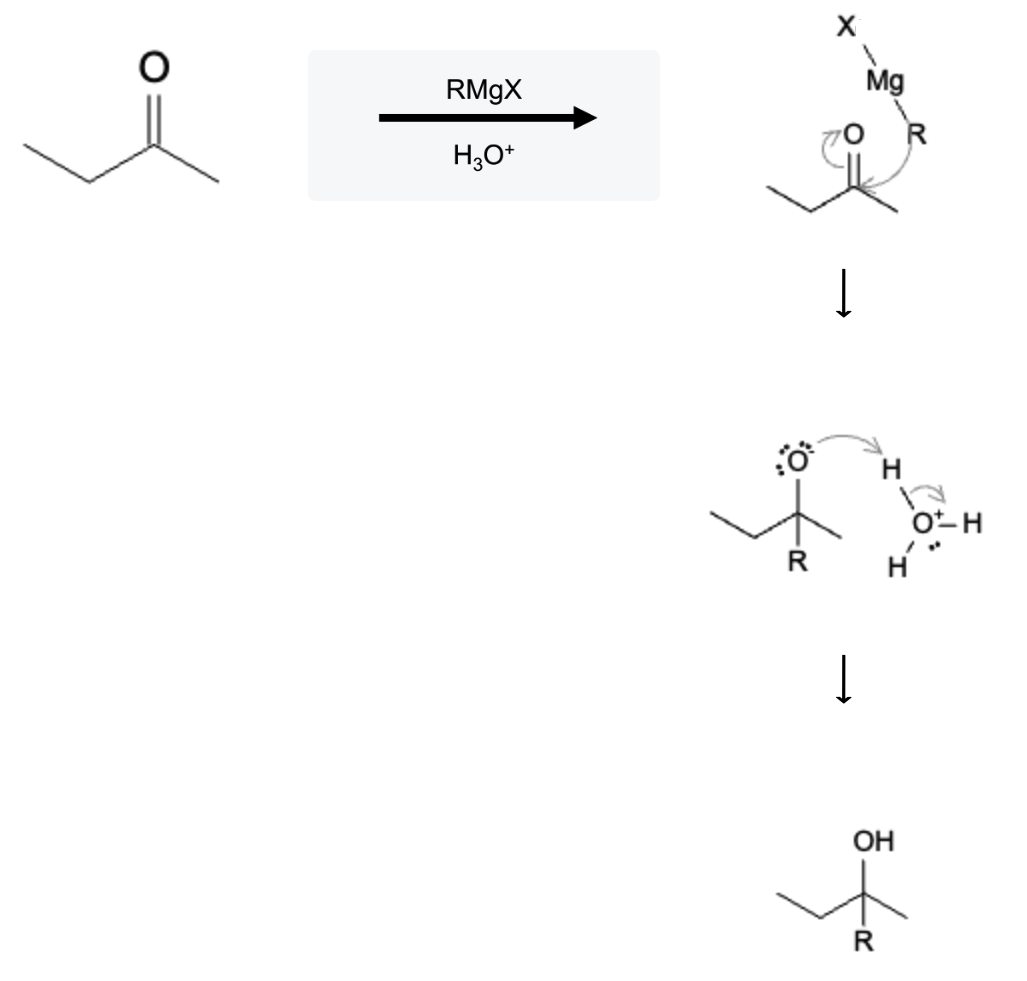
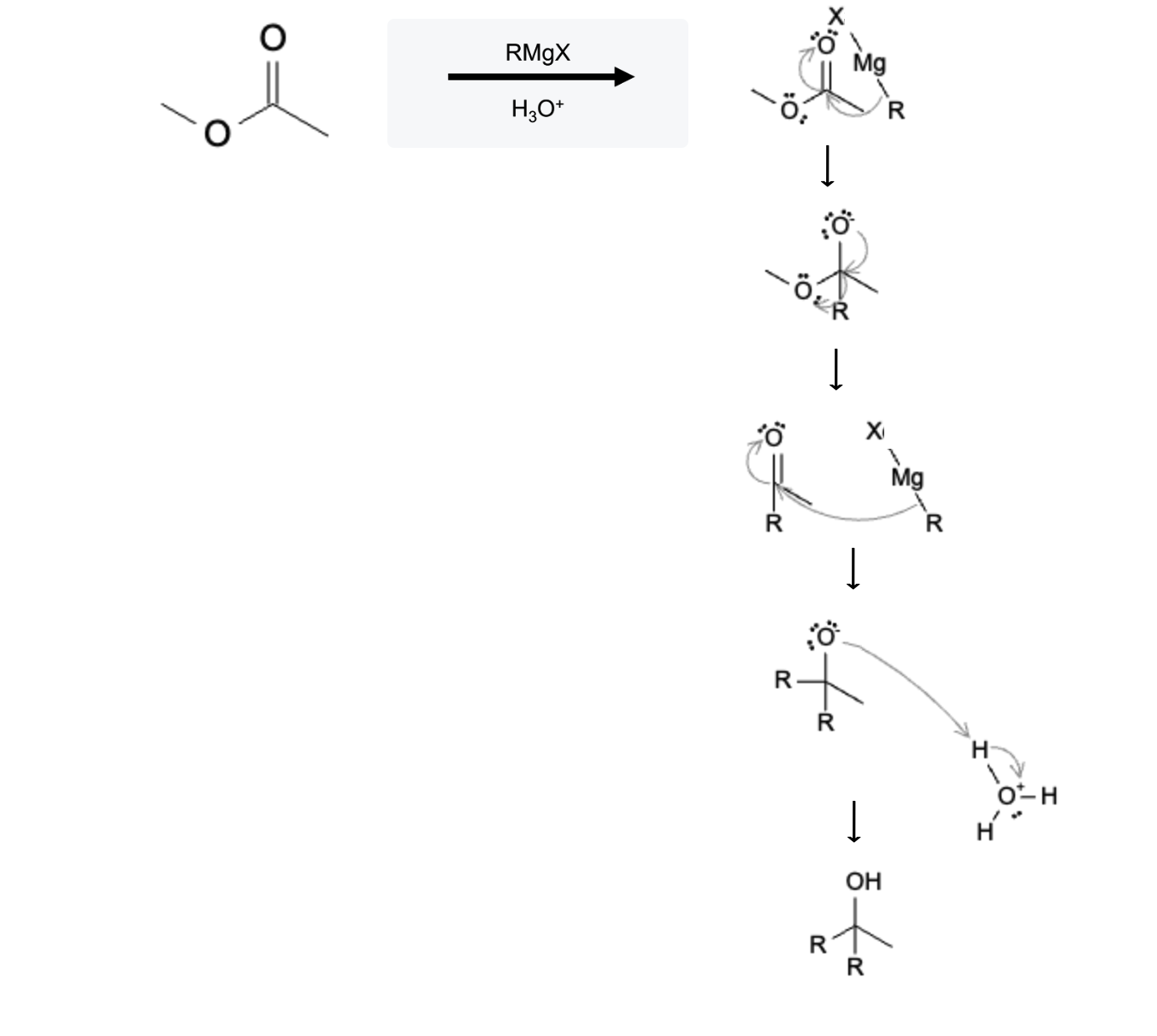
The reactivity of Grignard reagents and organolithium compounds stems from the polarity of the carbon-metal bond, where the carbon atom bears a partial negative charge. This electron-rich nature allows these compounds to act as powerful nucleophiles, attacking electrophilic centers in a variety of reactions. Their reactions include nucleophilic additions, reductions, and reactions with carbonyl compounds, among others. Both of these compounds react in the same fashion so for simplicity, Grignard reagents are shown below:
Grignard Carbonyl Reaction
Grignard Reduction of Ester
Applications in Organic Synthesis:
Grignard reagents and organolithium compounds have found widespread applications in organic synthesis due to their ability to form new carbon-carbon bonds. They can participate in reactions such as Grignard additions, where they add to electrophiles to form new carbon-carbon bonds. Additionally, they are valuable reagents in the synthesis of alcohols, acids, esters, and many other functional groups.
It is crucial to handle Grignard reagents and organolithium compounds with caution due to their reactivity and sensitivity to air and moisture. These compounds should be prepared and used under strictly controlled conditions, including the use of inert atmospheres and proper protective equipment. Adequate training and knowledge of safe handling procedures are essential when working with these potent reagents.
Gilman Reagents
Organocuprates, also known as organolithium cuprates or Gilman reagents, are formed by the reaction of organolithium compounds with copper(I) salts. These compounds consist of a copper atom coordinated to an alkyl or aryl group, as well as a negatively charged ligand, commonly a halide or alkoxide. The synthesis of organocuprates typically involves the reaction of an alkyl or aryl lithium compound with a copper(I) salt, such as copper(I) iodide. Functionally, this is a modification of an organolithium compound, taking 2 equivalents of an organolithium product and reacting with Cu-X to form R2CuLi:
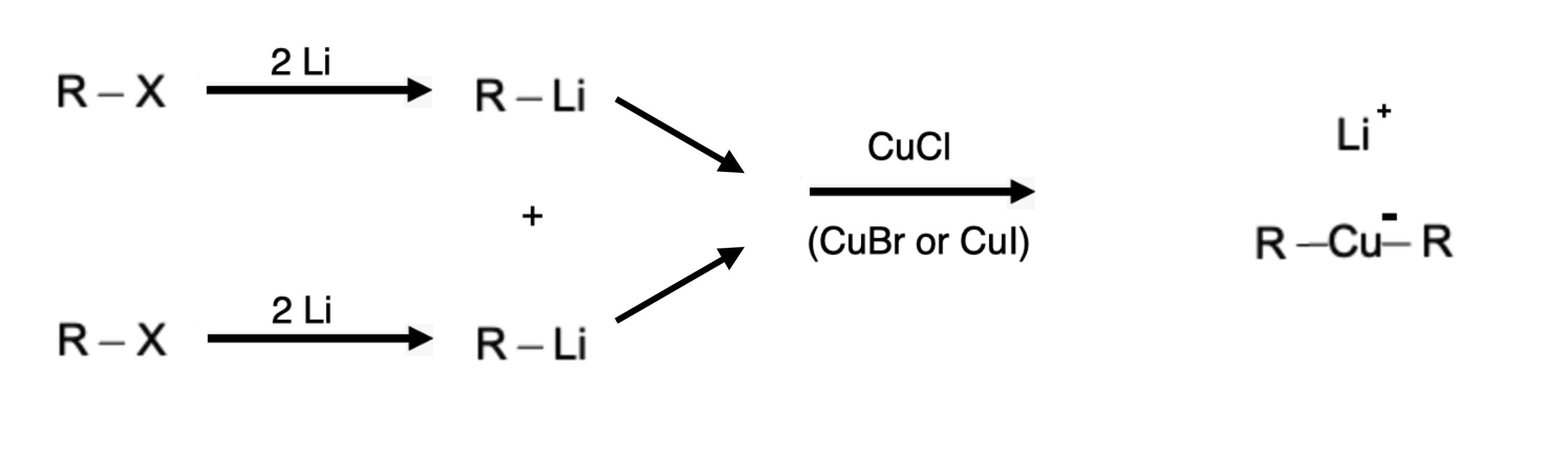
Grignard vs Gilman: Whats the difference?
Both compounds represent organometallic compounds that are highly reactive however, they each have their specific uses.
Grignard Reagent Reactions
Griganrd reagents will typically react with aldehydes, ketones, carboxylic acids, acid chlorides, anhydrides, esters epoxides, and nitriles.
Gilman Reagent Reactions
Gilman reagents will typically react with alkyl halides, alkenes, acid chlorides, esters, amides, and anhydrides.
Summary
Grignard reagents and organolithium compounds have emerged as vital warriors in the arsenal of organic chemists. Through their unique reactivity and carbon-metal bonds, they offer unprecedented opportunities for the construction of complex organic molecules. In this article, we explored the preparation, properties, reactivity, and applications of these organometallic compounds. By harnessing the power of Grignard reagents and organolithium compounds, scientists continue to advance the frontiers of organic synthesis, shaping the future of chemistry.
Test Your Knowledge:
What does a Grignard reagent look like?
What types of reactions do Grignard reagents participate in? What types of reactions do Gilman reagents participate in?