Enols and Enolates: Mastering Organic Chemistry Reactivity
Enols and Enolates: Mastering Organic Chemistry Reactivity
1. Introduction to Enols and Enolates
Enols and enolates are critical intermediates in organic chemistry, especially in reactions involving aldehydes, ketones, and related carbonyl compounds. Mastery of their behavior allows precise control over various synthesis pathways.
Defining Enols
An enol is a compound featuring a hydroxyl group (–OH) bonded directly to an alkene carbon (C=C). The term "enol" combines the functional groups alkene (en-) and alcohol (-ol). For most carbonyl compounds, enol forms exist in equilibrium with keto forms, a process known as keto-enol tautomerization.
Defining Enolates
An enolate is the conjugate base of an enol, typically generated by deprotonating the α-carbon (carbon adjacent to the carbonyl) of aldehydes or ketones. Enolates are highly nucleophilic and serve as pivotal intermediates in C–C bond-forming reactions.
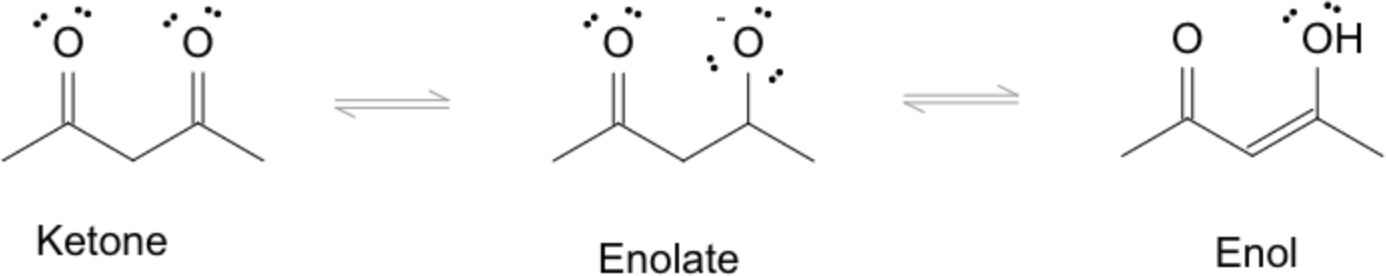
2. Keto-Enol Tautomerization
Keto-enol tautomerization is the rapid equilibrium between keto (carbonyl-containing) and enol forms.
- Acid-Catalyzed Mechanism: Protonation of carbonyl oxygen, followed by deprotonation at α-carbon, followed by protonation at the oxygen.
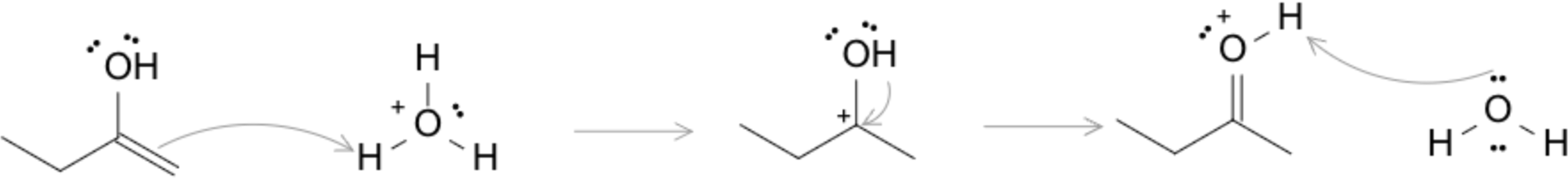
- Base-Catalyzed Mechanism: Deprotonation at the enol -OH, followed by enolate intermediate formation, followed by protonation at α-carbon.

Importance in Organic Synthesis
Understanding keto-enol tautomerism is crucial as the equilibrium position significantly affects reaction outcomes. Typically, keto forms predominate due to their greater thermodynamic stability.
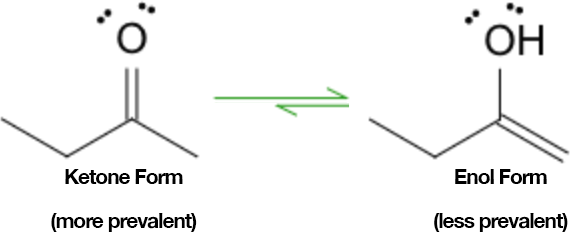
Keto–enol tautomerism: the ketone form is usually the major tautomer under standard conditions.
3. Formation and Stability of Enolates
Enolate generation is commonly performed using strong bases. Key bases include:
- LDA (lithium diisopropylamide): Generates kinetic enolate (less-substituted, formed at low temperatures, –78 °C).
- NaH or NaOH: Generates thermodynamic enolate (more substituted, favored under equilibrating conditions).
- KOt-Bu: Bulky base favoring less-substituted enolate formation due to steric hindrance.
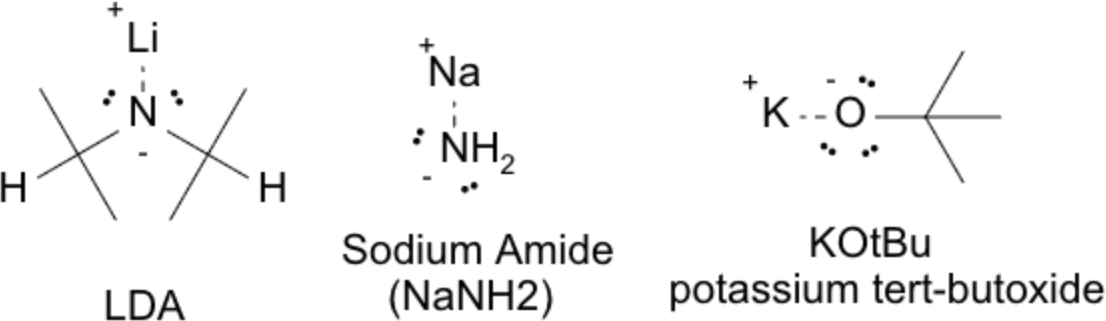
Structures of three commonly used strong bases in organic synthesis: LDA (lithium diisopropylamide, a non-nucleophilic base), NaNH₂ (sodium amide, very strong base), and KOtBu (potassium tert-butoxide, a bulky base that favors elimination).
Kinetic vs Thermodynamic Enolates
- Kinetic Enolate: Forms fastest, at lower temperatures with bulky, non-nucleophilic bases (LDA).
- Thermodynamic Enolate: More stable, forms slowly at higher temperatures and equilibrating conditions (NaOH or NaOMe).
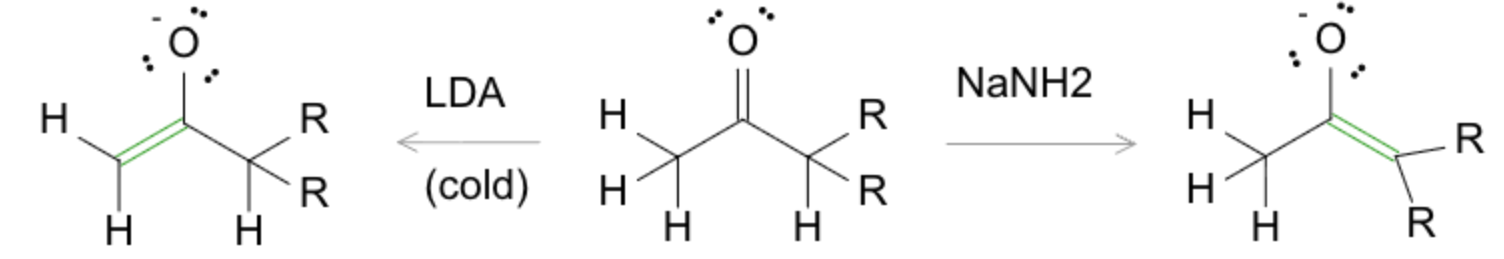
Exam tip: if the conditions are bulky base + low temperature (e.g., LDA, −78 °C), expect the kinetic (less-substituted) product; if conditions allow equilibration (small base, higher temperature, protic solvent, e.g., NaNH2, room temp), expect the thermodynamic (more-substituted) product
4. Important Reactions Involving Enols and Enolates
α-Halogenation
- Enols or enolates react readily with halogens (Br₂, Cl₂, I₂) at the α-position, forming α-halo carbonyl compounds:
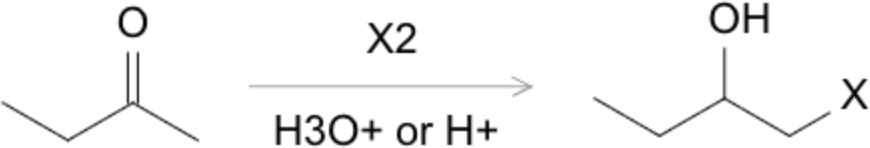
Alkylation of Enolates
- Enolates are nucleophilic and react with primary alkyl halides (R–X) in SN2 reactions to form C–C bonds.

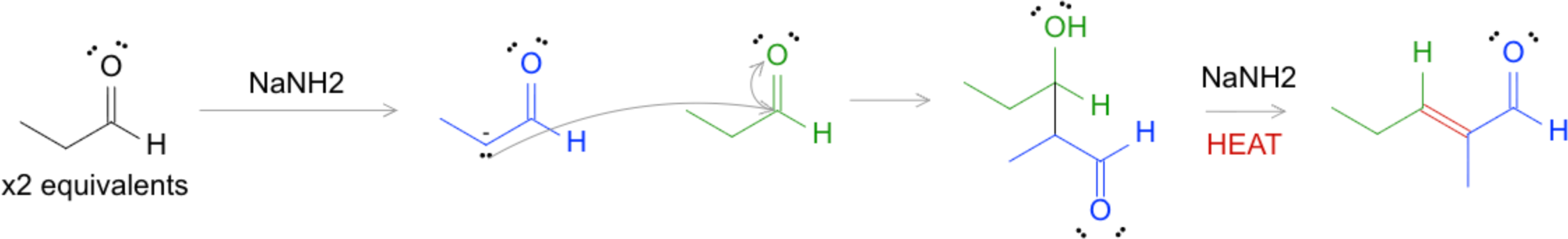
Aldol Reaction and Condensation
- In the aldol reaction, an enolate ion attacks another carbonyl compound, forming β-hydroxy aldehydes or ketones.
- Heating these products leads to aldol condensation, generating α,β-unsaturated aldehydes or ketones.
Claisen Condensation
- Ester enolates react with esters or ketones in base (usually NaOEt) to yield β-keto esters or β-diketones.

5. Summary and Key Takeaways
- Enols and enolates are fundamental intermediates for diverse organic reactions.
- Kinetic vs. thermodynamic control is a critical concept in manipulating enolate chemistry.
- Enolates participate prominently in reactions like aldol condensations, Michael additions, and Claisen condensations.