Isomerism of Alkanes: Chain vs. Positional Isomers
Types of Isomers (Structural vs Stereoisomers)
Isomerism explains how one molecular formula can produce multiple structures with different properties. Broadly, isomers split into structural (constitutional) isomers and stereoisomers. Knowing whether atoms change connectivity or only their 3D arrangement is the key to classifying them.
Structural (Constitutional) Isomers
Structural isomers share a formula but differ in how atoms are bonded. Changes in connectivity can swap chain length, branching, or functional groups, yielding molecules with distinct shapes and properties.
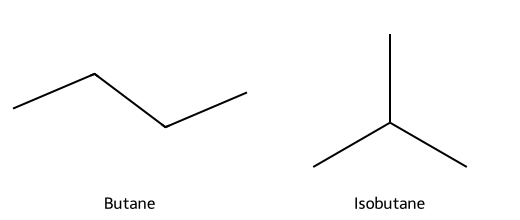
- Example: C₄H₁₀ exists as n-butane (straight chain) and isobutane (branched 2-methylpropane). Same formula, different connectivity → different boiling points and shapes.
- As formulas grow larger, the number of possible constitutional isomers rises quickly (e.g., alcohol vs ether for the same formula).
Stereoisomers
Stereoisomers keep the same connectivity but arrange groups differently in space. This 3D difference can profoundly affect reactivity and biological activity.
Subtypes:
- Enantiomers: Non-superimposable mirror images.
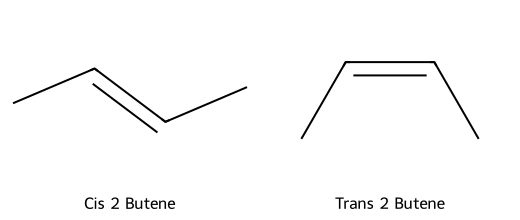
- Diastereomers: Stereoisomers that are not mirror images (includes cis/trans alkenes or rings, molecules with multiple stereocenters that differ at some but not all centers).
- Conformational isomers: Same connectivity, interconvert by bond rotation (often treated separately because they interconvert readily).
Quick Comparison
- Structural isomers: different atom connections → often different functional groups or branching.
- Stereoisomers: same connections, different 3D arrangement → enantiomers, diastereomers (including cis/trans), and conformers.
Summary
Isomers with the same formula diverge in two ways: connectivity changes give structural (constitutional) isomers, while 3D arrangement changes give stereoisomers. Distinguishing between these classes underpins naming, predicting properties, and understanding reactivity.