Physical Properties of Alkanes and Cycloalkanes
Physical Properties of Alkanes and Cycloalkanes
Alkanes and cycloalkanes are nonpolar hydrocarbons whose physical properties depend on size, shape, and ring constraints. Dispersion forces dominate, so boiling/melting trends track molecular surface area and compactness.
Boiling and Melting Point Trends
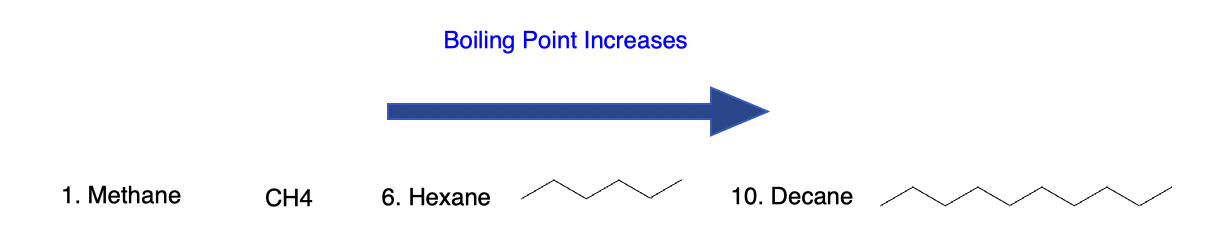
- Chain length: longer chains → higher boiling/melting points (stronger dispersion).
- Branching: lowers boiling point (more compact, less surface contact); often raises symmetry which can raise melting point.
- Example: n-pentane (36 °C) > isopentane (27 °C) > neopentane (9.5 °C) for boiling points.
Solubility and Density
- Solubility: insoluble in water; soluble in nonpolar/weakly polar solvents.
- Density: < 1 g/mL; liquids float on water.
- Small alkanes gases at RT; mid-length liquids; very long chains waxy solids.

Cycloalkane Comparisons
- Rings reduce flexibility; similar C count vs open chain often gives slightly higher boiling/melting points (tighter packing, larger surface).
- Chair cyclohexane is strain-free with ~109.5° angles; ring size and substituent orientation influence stability/physical data.
Summary
- Dispersion forces control alkane/cycloalkane physical behavior.
- Longer chains ↑ boiling/melting; branching ↓ boiling (often ↑ symmetry for melting).
- Nonpolar, low-density, water-insoluble; cycloalkanes often boil/melt slightly higher than their open-chain counterparts.