Conformational Analysis and Stability
Conformational Analysis and Stability
Conformations arise from rotation about single (σ) bonds. Conformational analysis compares these 3D arrangements to explain which shapes are lowest in energy. Newman projections are the standard way to visualize and rank conformations in simple alkanes.
Newman Projections and Dihedral Angles
Look down a C–C bond axis: the front carbon is a dot with three bonds, while the rear carbon is a circle with three bonds radiating outward. The dihedral angle is measured between one front bond and one rear bond.
Staggered conformations (≈60°) spread torsional strain out, while eclipsed conformations (0°) align bonds and increase strain.
Sources of Strain: Torsional vs Steric
- Torsional strain: arises when bonds eclipse, bringing electron clouds close together.
- Steric strain: stems from bulky groups crowding even in staggered forms (e.g., methyl groups in gauche butane).
In ethane, torsional strain dominates; in butane and larger alkanes, both torsional and steric strain shape the energy profile.
Ethane: Baseline Rotational Barrier
Ethane’s staggered conformation is roughly 3.0 kcal·mol⁻¹ lower in energy than its eclipsed form. At room temperature the molecule rotates quickly, but it still spends most of its time in staggered arrangements because they minimize torsional strain between aligned C–H bonds.
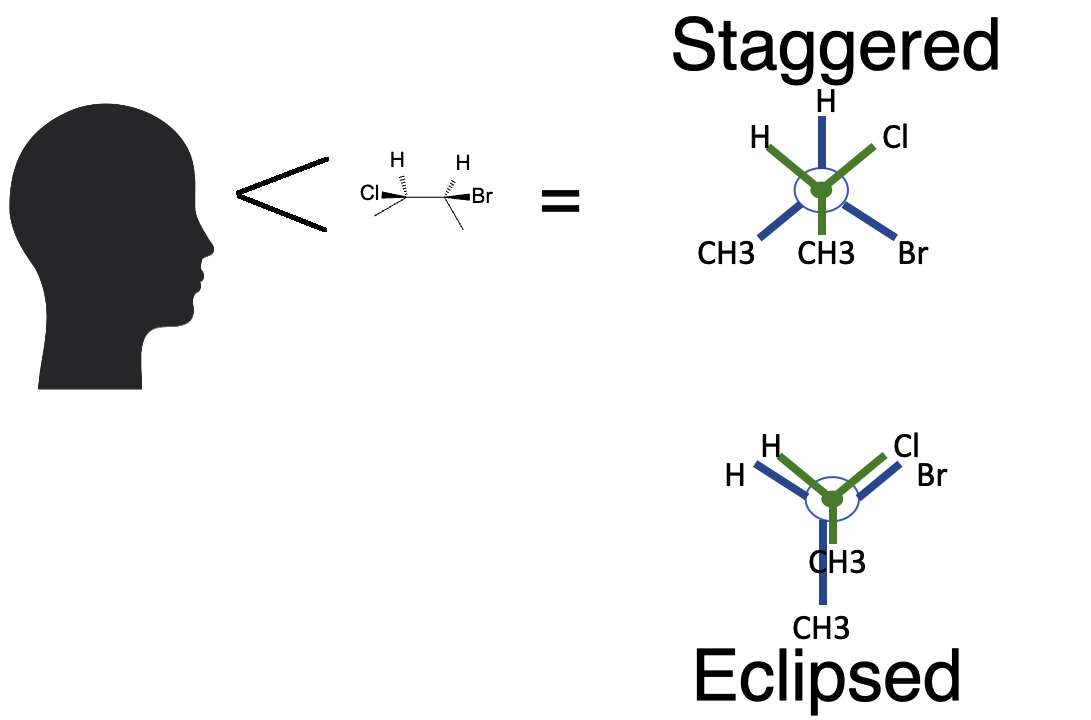
Butane: Anti, Gauche, and Eclipsed
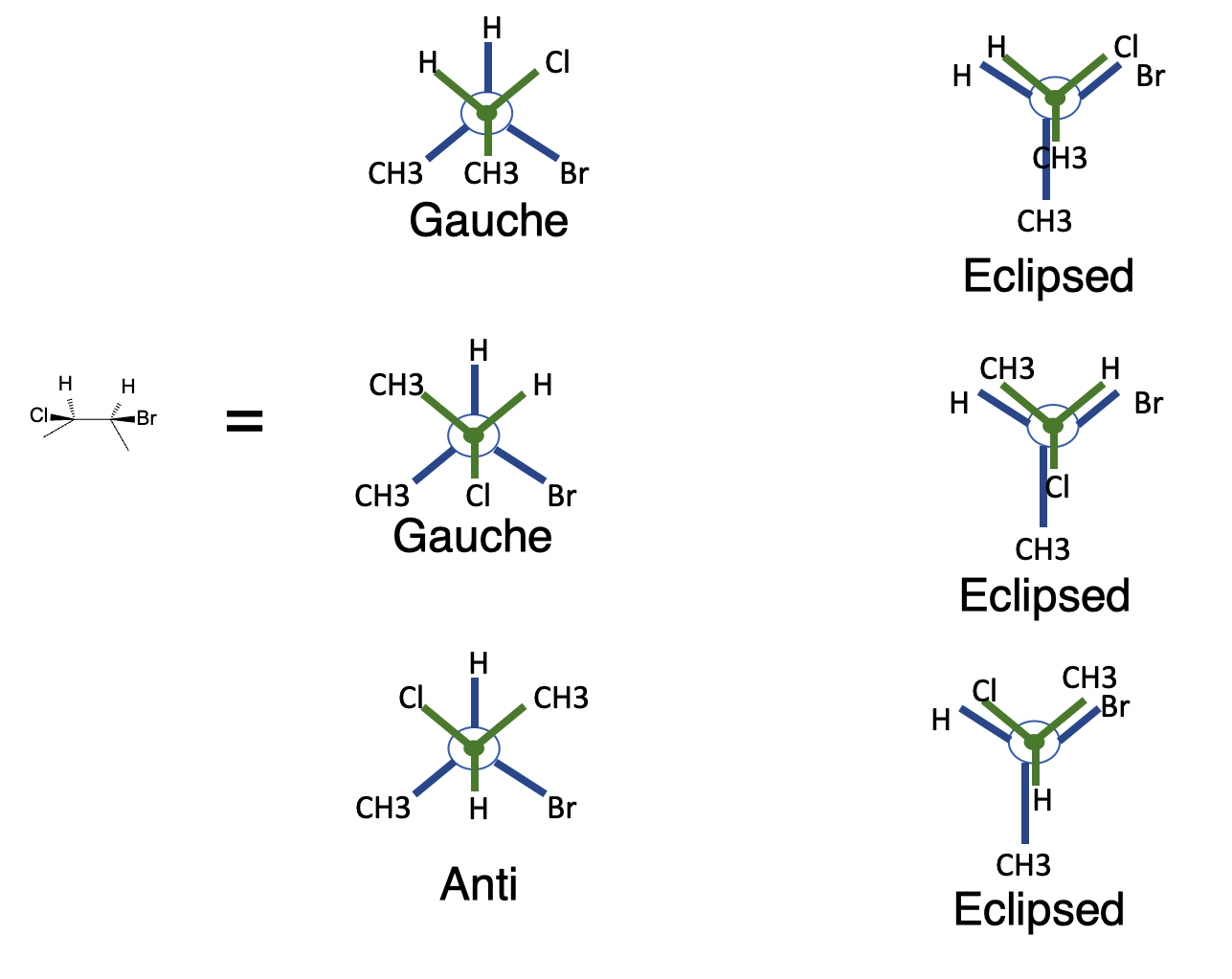
Rotation around the central C2–C3 bond in butane introduces steric strain because two methyl groups are present. Key conformers:
- Anti (staggered, 180° methyl dihedral): methyl groups opposite → lowest energy and dominant population.
- Gauche (staggered, 60°): methyl groups adjacent → ~0.9 kcal·mol⁻¹ higher due to steric crowding (~15–20% population).
- Eclipsed (Me–H or Me–Me): significantly higher energy; fully eclipsed (Me–Me) combines torsional and steric strain and is essentially negligible.
Energy Snapshot for Butane (qualitative)
| Conformation | Description | Relative Energy | Population |
|---|---|---|---|
| Anti staggered | Methyls 180° apart | Lowest | Major (~80%) |
| Gauche staggered | Methyls 60° apart | + steric penalty | Minor (~15–20%) |
| Eclipsed (Me–H) | Methyl eclipses hydrogen | High torsional strain | Trace |
| Eclipsed (Me–Me) | Methyl eclipses methyl | Highest torsional + steric | Negligible |
Why Conformational Preferences Matter
Preferred conformers influence:
- E2 reactions, which demand anti‑periplanar alignment of leaving group and β-hydrogen.
- Equilibria in substituted systems, where steric crowding pushes populations toward less strained conformers.
- Spectra, since NMR averages over populated conformations.
Summary
- Newman projections reduce 3D rotations to clear dihedral views.
- Staggered conformations minimize torsional strain; anti is best in butane, followed by gauche, then eclipsed forms.
- These concepts support later discussions of cyclohexane chairs, stereoselective synthesis, and elimination reactions.