Synthesis of Alkynes
Synthesis of Alkynes
Two workhorse strategies dominate alkyne construction: double eliminations from dihalides and acetylide alkylations after deprotonating a terminal alkyne. Together they let you either create the triple bond or extend the carbon chain.
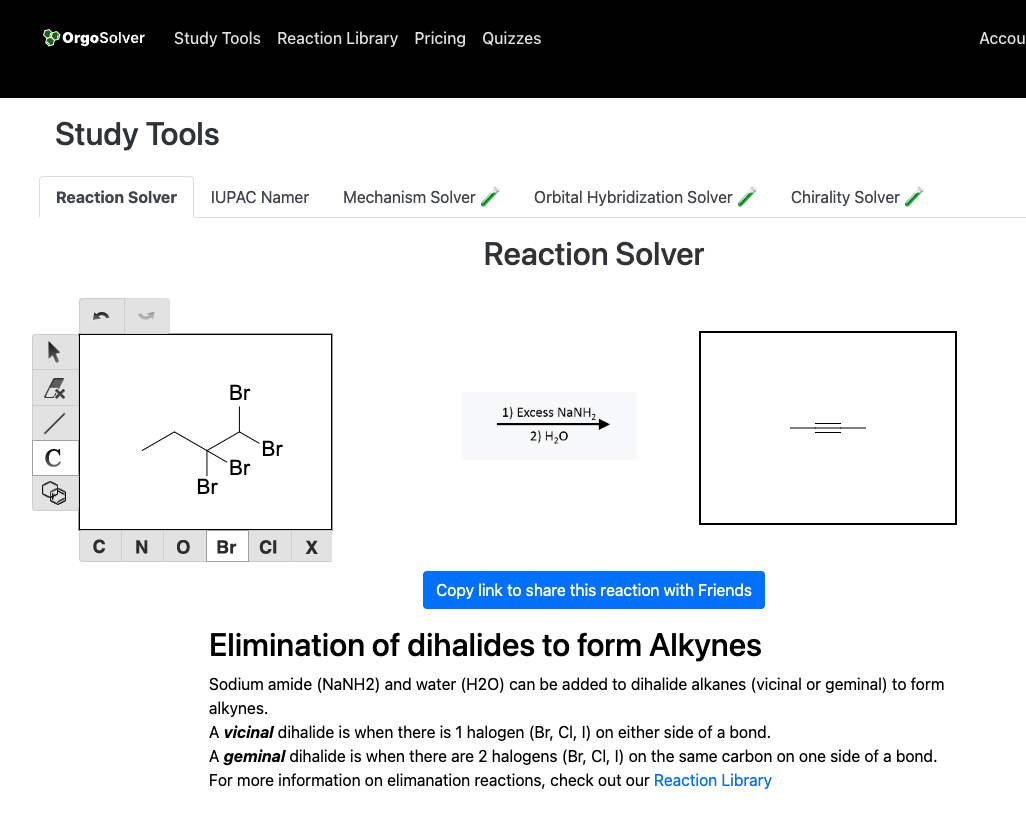
Double dehydrohalogenation
- Start from a vicinal or geminal dihalide and treat with excess strong base (classically NaNH₂ in liquid NH₃).
- Two consecutive E2 eliminations remove HX twice to give the alkyne (often via a vinyl halide intermediate).
- Terminal targets usually need 3 equiv base: two for eliminations, one to deprotonate the terminal alkyne (then protonate in workup).
Tips
- Use vicinal/geminal dihalides; strong base and cold temps favor clean eliminations.
- If you want a terminal alkyne, quench the acetylide with water/acid after the eliminations.
Acetylide formation and SN2 alkylation
- Deprotonate a terminal alkyne (pKₐ ~25) with a strong base (NaNH₂, NaH, n-BuLi) to make an acetylide.
- Perform SN2 on a methyl or primary alkyl halide (or tosylate) to extend the carbon chain.
- Secondary/tertiary halides give elimination instead of substitution.
Workflow
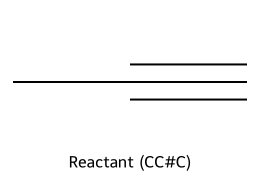
- Terminal alkyne + base → acetylide.
- Acetylide + 1° (or Me) RX → new C–C bond (internal alkyne if you alkylate twice from acetylene).
- Use aprotic solvents (THF, DMSO, DMF) and exclude water to avoid protonation.
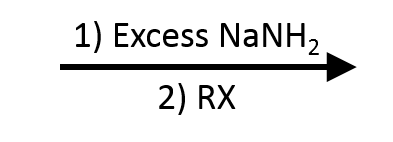

Summary
- Build the triple bond: Double E2 of vicinal/geminal dihalides with strong base.
- Extend the chain: Deprotonate a terminal ≡C–H and SN2 on 1°/Me electrophiles.
- Control stoichiometry: extra base is needed when targeting a terminal alkyne; quench after to obtain the neutral product.