Diastereomers and Meso Compounds
Diastereomers and Meso Compounds
Diastereomers are stereoisomers that are not mirror images. They share connectivity but differ in 3D arrangement without being enantiomeric. Meso compounds are a special achiral case within multi-center systems.
What Are Diastereomers?
- Cis/trans pairs of alkenes or rings (e.g., cis vs trans 1,2-dichloroethene; cis vs trans disubstituted cycloalkanes).
- Molecules with multiple stereocenters that differ at some, but not all, centers (e.g., (R,R) vs (R,S)).
- Diastereomers have different physical properties (bp, mp, solubility), making separation easier than enantiomer resolution.
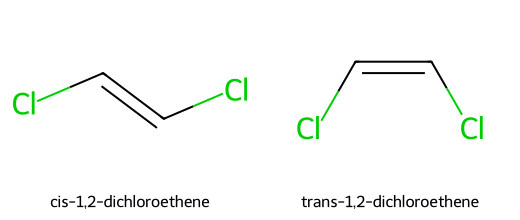
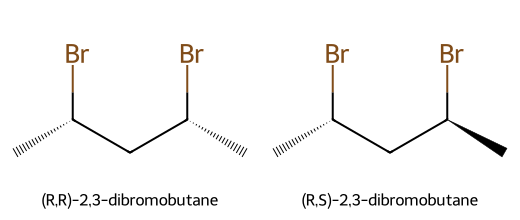
Meso Compounds
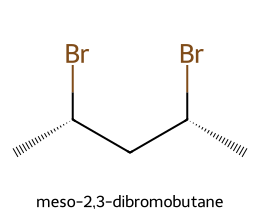
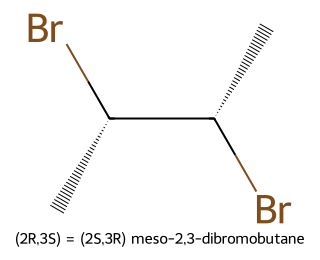
Meso compounds contain stereocenters yet are overall achiral because of an internal symmetry plane (or center). Classic example: meso-2,3-dibromobutane—two chiral centers, but an internal mirror plane makes the whole molecule achiral and optically inactive.
Key points:
- Meso forms are diastereomeric to the chiral stereoisomers of the same formula.
- They do not have enantiomeric partners (they are their own mirror image).
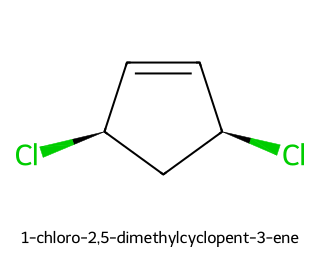
(1R,4R)-1,4-dichlorocyclopent-2-ene — meso due to the internal mirror plane
Counting and Classifying
- For n stereocenters, max stereoisomers = 2ⁿ minus any meso reductions.
- Identify internal symmetry (Fischer projections help) to spot meso cases and adjust counts.
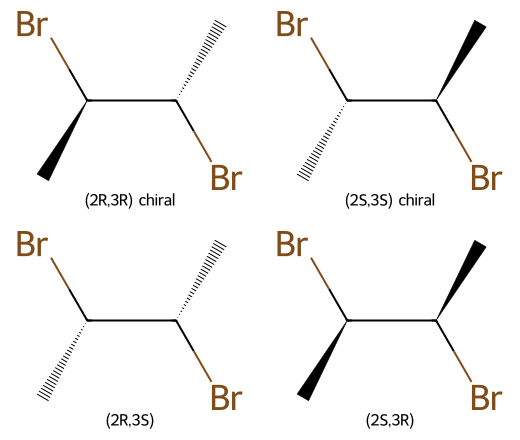
Worked example: 2,3-dibromobutane (n = 2)
- Max from 2ⁿ: 2² = 4 formal configurations: (2R,3R), (2S,3S), (2R,3S), (2S,3R).
- (2R,3R) and (2S,3S) are enantiomers.
- (2R,3S) and (2S,3R) collapse into one meso stereoisomer (internal mirror plane).
- Actual distinct stereoisomers = 4 − 1 meso = 3.
Summary
Diastereomers are stereoisomers that aren’t mirror images—cis/trans pairs or multi-center molecules differing at one or more (but not all) stereocenters. Meso compounds are symmetry-driven exceptions: stereocenters present, but overall achiral and optically inactive. Recognizing these relationships is essential for predicting stereoisomer counts and separating real-world mixtures.