Fischer Projections and 3D Representation
Fischer Projections and 3D Representation
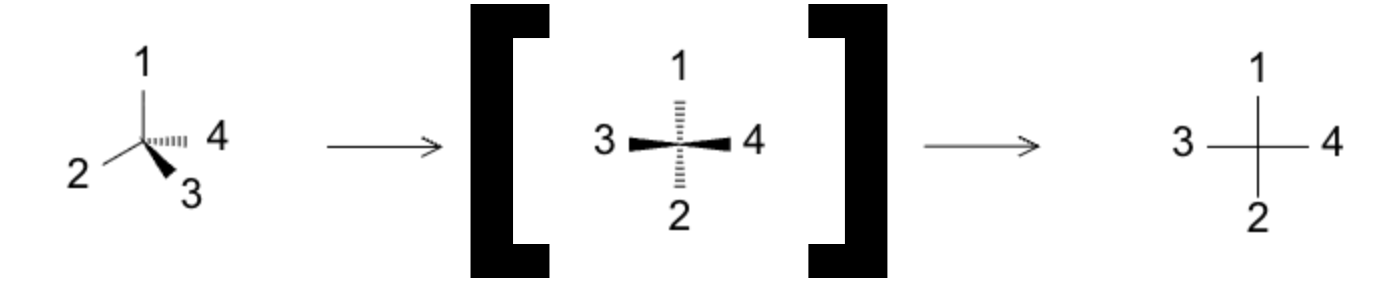
Fischer projections flatten 3D chiral molecules into a cross while retaining stereochemical meaning—horizontal bonds come out, vertical bonds go back. Widely used for sugars and amino acids, but applicable to any stereocenter chain.
Reading a Fischer
- Chiral center sits at the cross intersection.
- Horizontal = toward you (wedge-like), vertical = away (dash-like).
- Main chain usually vertical with the most oxidized carbon at the top (e.g., aldose sugars).
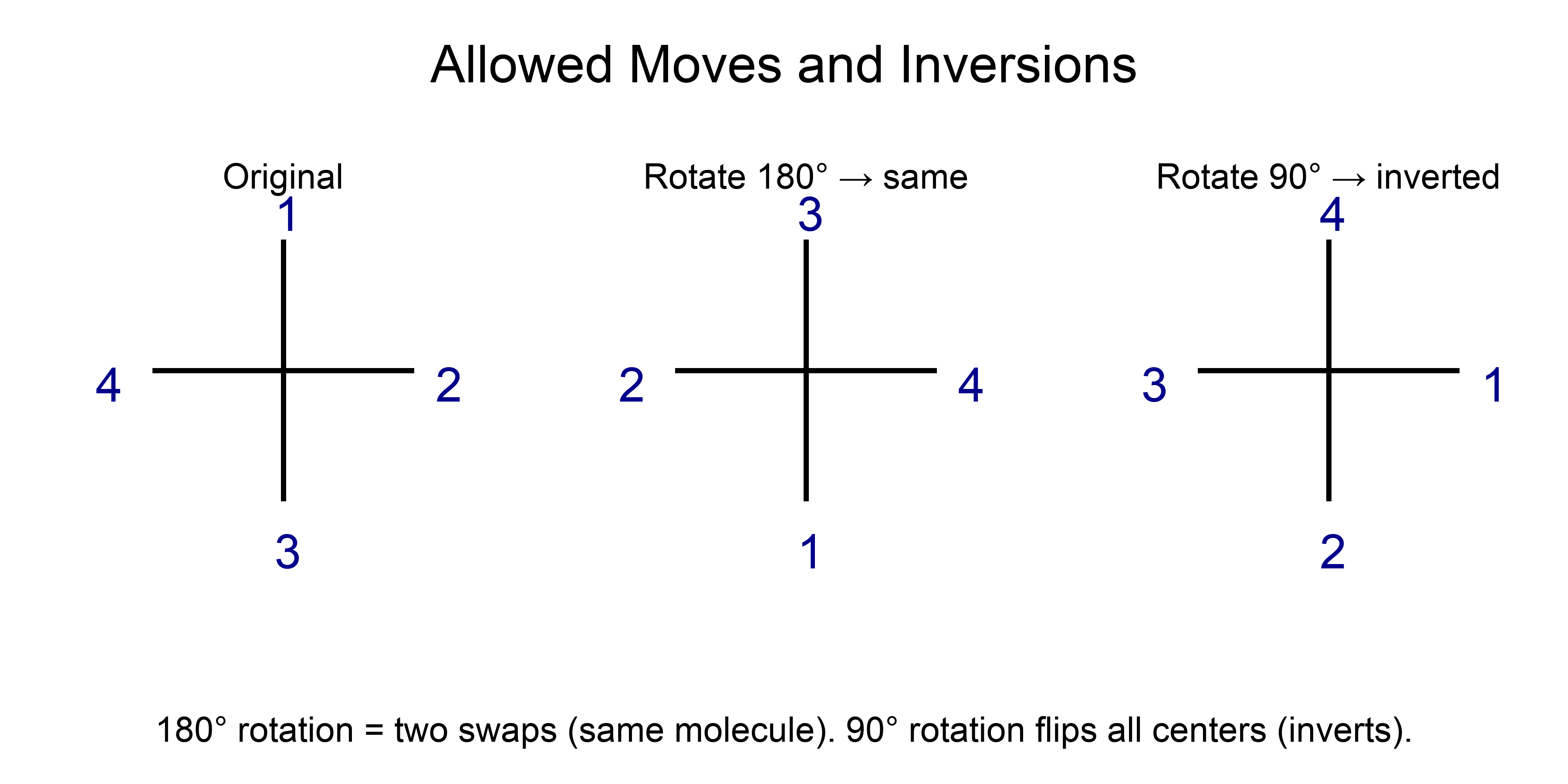
Allowed Moves and Inversions
- 180° rotation in the plane → same molecule (equivalent to two swaps).
- 90° rotation → inverts all centers (invalid if you intend the same structure).
- Swapping any two substituents at a center inverts that center; two swaps restore it.
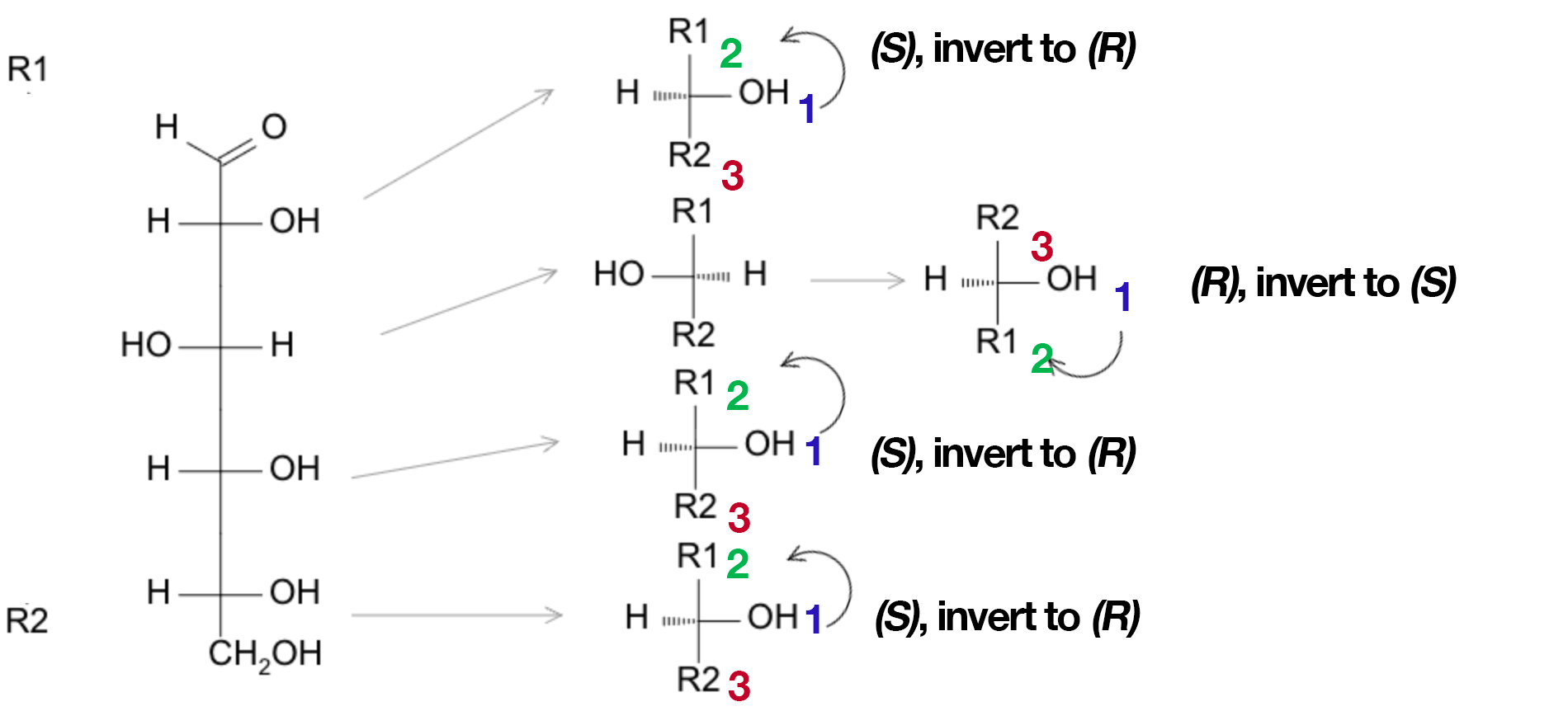
Assigning R/S from a Fischer (Quick Trick)
- Put the lowest priority on a vertical position (already pointing away), then trace 1 → 2 → 3 (CIP): clockwise = R, counterclockwise = S.
- If the lowest priority sits on a horizontal bond (toward you), assign as seen then invert the result.
Why Use Fischers
- Rapid comparison of multiple stereocenters (e.g., sugar series).
- Easy detection of meso symmetry in multi-center systems.
- Compact way to communicate stereochemistry without full 3D wedges.
Summary
In a Fischer, horizontal = out, vertical = in. Only 180° rotations keep the same molecule; 90° flips invert it. With CIP priorities, you can assign R/S directly. Mastery of Fischer projections speeds up stereochemical comparisons and naming.
