Enantiomers and diastereomers
Stereochemistry is the branch of chemistry that studies the three-dimensional arrangement of atoms in molecules and their effect on chemical and physical properties. Chirality is a fundamental concept in stereochemistry, and it describes the property of a molecule to exist in two mirror-image forms that are not superimposable on each other, called enantiomers. In this lesson, we will discuss enantiomers and diastereomers, the two main types of stereoisomers.
Enantiomers
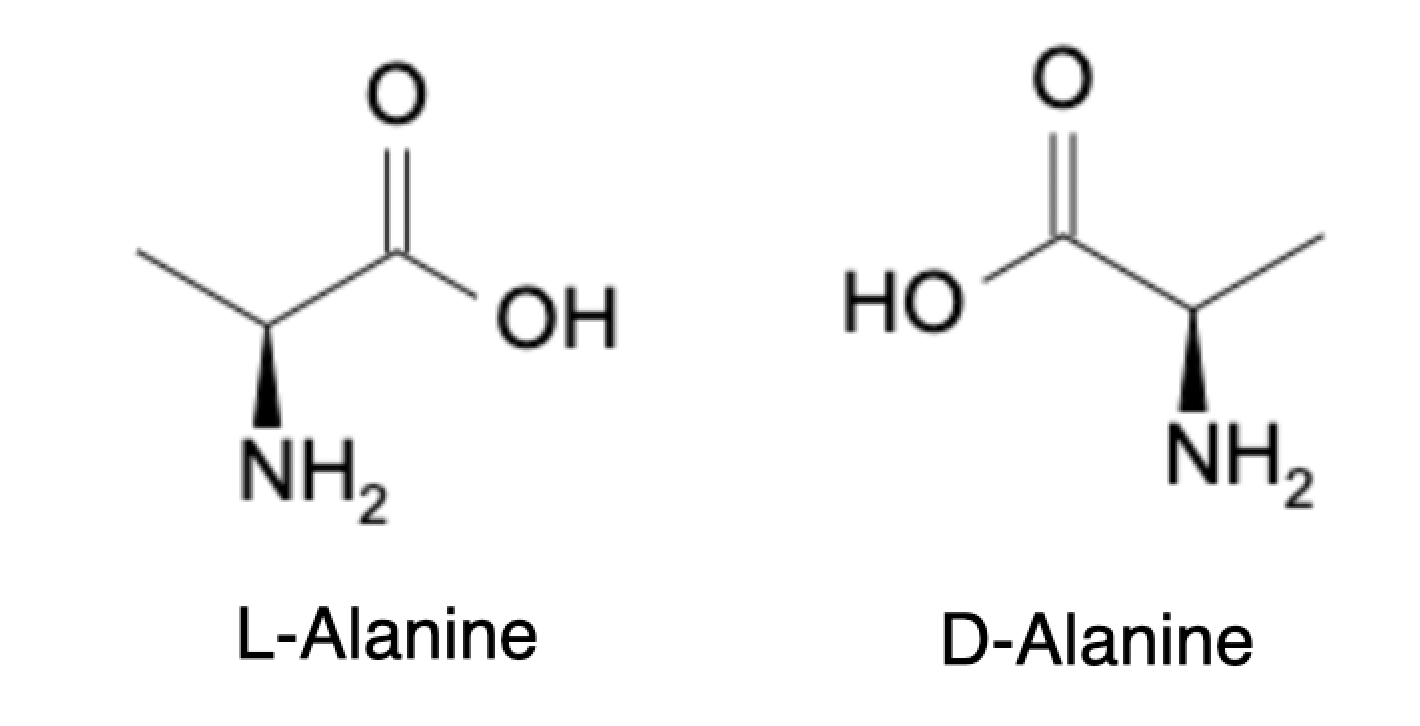
Enantiomers are stereoisomers that are mirror images of each other and cannot be superimposed onto each other. Enantiomers have identical physical and chemical properties except for their effect on plane-polarized light, a property known as optical activity. One enantiomer rotates plane-polarized light clockwise, while the other rotates it counterclockwise. This property is known as optical rotation and can be measured using a polarimeter. Enantiomers have the same boiling point, melting point, solubility, and density, but they can differ in their biological activity, taste, and smell. One example of enantiomers is L-alanine and D-alanine, which are mirror images of each other but have different optical rotations.
Diastereomers
Diastereomers are stereoisomers that are not mirror images of each other and can be distinguished by their physical and chemical properties. Diastereomers have different boiling points, melting points, solubilities, and densities. Unlike enantiomers, diastereomers can have different biological activities, taste, and smell. One example of diastereomers is cis- and trans-2-butene. These two isomers have different boiling points, and their reactivities are also different.

Stereochemistry
The stereochemistry of a molecule is determined by the presence of one or more stereocenters, which are atoms that are connected to four different substituents. Stereocenters can exist in two configurations, R and S, which are determined by the Cahn-Ingold-Prelog priority rules. In the R configuration, the highest priority substituent is on the right side, while in the S configuration, the highest priority substituent is on the left side. The configuration of a stereocenter determines the absolute configuration of the molecule and its stereochemistry.
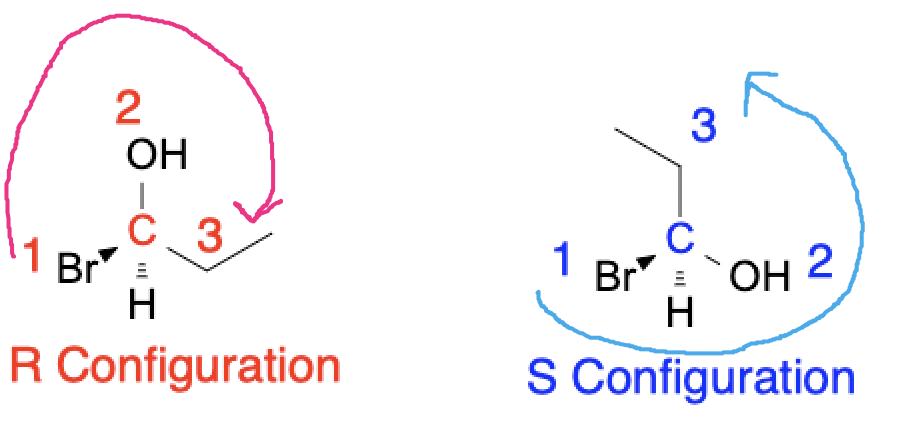
Check out our Chirality Solver to draw any molecule and identify if it is R or S configuration!
Summary
Enantiomers and diastereomers are two types of stereoisomers that play a crucial role in stereochemistry. Enantiomers are mirror images of each other and have identical physical and chemical properties except for their effect on plane-polarized light. Diastereomers are stereoisomers that are not mirror images of each other and can be distinguished by their physical and chemical properties. Stereochemistry is determined by the presence of stereocenters and their configuration, which is determined by the Cahn-Ingold-Prelog priority rules.
Test Your Knowledge:
What is the difference between enantiomers and diastereomers?
What would be the configuration of the molecule below: