Aldehyde/Ketone → Alcohol with Grignard (RMgBr, then H₃O⁺)
Grignard reagents RMgBr (prepared in dry Et₂O/THF) behave as carbanion equivalents: they attack aldehydes and ketones by 1,2‑addition to make magnesium alkoxides, which are protonated in a separate acidic workup (H₃O⁺) to furnish alcohols. Product class follows a strict rule—formaldehyde gives primary alcohols, other aldehydes give secondary alcohols, and ketones give tertiary alcohols. The reaction fails in the presence of water, acids, or unprotected protic groups because RMgBr is immediately quenched.
Quick Summary
- Reagents/conditions: RMgBr (1.0–1.5 eq) in dry Et₂O/THF at 0 → rt under inert gas; then separate H₃O⁺ quench.
- Outcome rule: HCHO → 1° alcohol, R′CHO → 2° alcohol, R′COR″ → 3° alcohol.
- Mechanism: Polar 1,2‑addition forms a magnesium alkoxide; acidic workup protonates the O⁻.
- Stereochemistry: New stereocenters form racemically (planar C=O → attack from either face) unless chiral control is specified.
- Selectivity: Grignards are “hard” nucleophiles (1,2‑addition). Use cuprates (R₂CuLi) for conjugate 1,4‑addition to α,β‑unsaturated carbonyls.
- Compatibility: Moisture, protic groups (–OH, –NH, –CO₂H), or CO₂ will quench RMgBr—protect or avoid these before addition.
- Depiction note: Figures show RMgBr with bromide as the representative halide; any RMgX behaves the same way.
Mechanism — 1,2‑Addition → Magnesium Alkoxide → H₃O⁺ Workup (3 Steps)
Scope & Limitations
- Works well: Formaldehyde, aliphatic or aromatic aldehydes, and ketones lacking acidic functionality; alkyl, vinyl, or aryl Grignards.
- Slower/problematic: Highly hindered ketones, substrates containing strong Lewis bases that chelate Mg and slow addition, or carbonyls adjacent to acidic/protic sites.
- Incompatible: –OH, –NH, –CO₂H, water, or CO₂ (they protonate or react with RMgBr). Protect alcohols/phenols as silyl ethers; convert acids to esters/amides first.
- Not this manifold: Esters or acid chlorides undergo multiple additions with RMgBr (tertiary alcohols via two additions). Use Gilman reagents for selective 1,4‑addition to enones.
Edge Cases & Exam Traps
- Adding acid too soon: Any acid present during the addition step destroys RMgBr. Acid appears only in the separate quench.
- Hidden proton sources: Wet glassware, protic solvents, or ammonium salts instantly quench Grignards. Dry everything.
- CO₂ contamination: RMgBr + CO₂ → carboxylates (a different reaction). Keep CO₂ out unless carboxylation is intended.
- Stereochemical claims: Planar C=O → racemic products. Claiming single enantiomers without chiral induction is incorrect.
- Conjugation confusion: For α,β‑unsaturated carbonyls, RMgBr gives 1,2‑addition; 1,4 requires R₂CuLi (Gilman). A favorite comparison question.
Practical Tips
- Flame-dry glassware and use dry Et₂O/THF under N₂/Ar. Keep RMgBr free of moisture and air.
- Add carbonyl slowly to the cooled Grignard solution to manage exotherms; allow to warm only after addition is complete.
- Quench at 0 °C with saturated NH₄Cl or dilute H₃O⁺; add aqueous acid slowly to avoid violent protonation.
- Protect alcohol/phenol groups (e.g., TBS ethers) and sequence reactions so the Grignard step happens before exposing those groups again.
- If no reaction occurs, confirm reagent freshness, dryness, and consider activating very hindered ketones with Lewis acids (advanced strategy).
Worked Examples
Reactant
Reagent
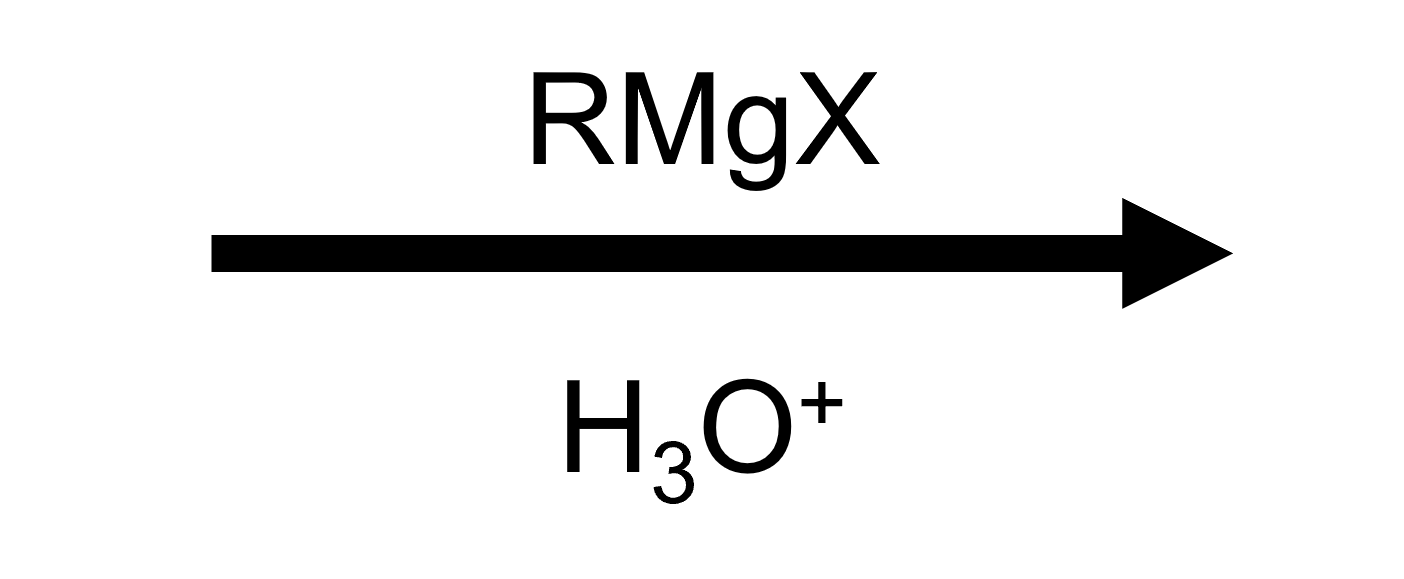
Product
Reactant
Reagent
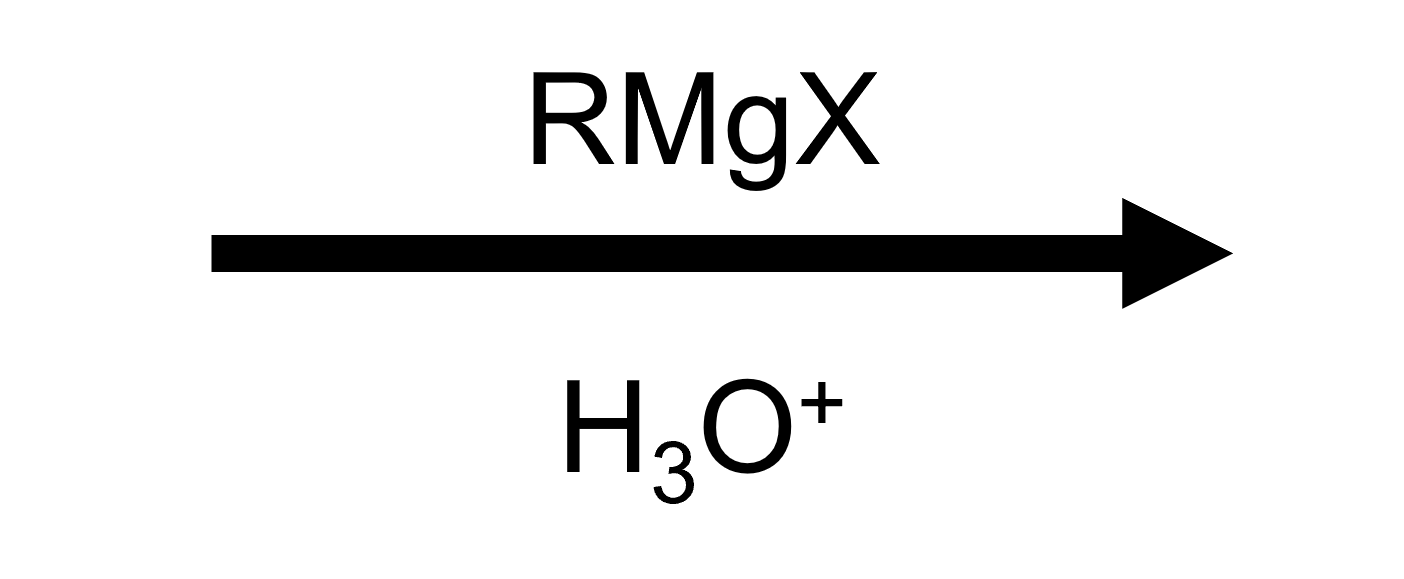
Product
Reactant
Reagent
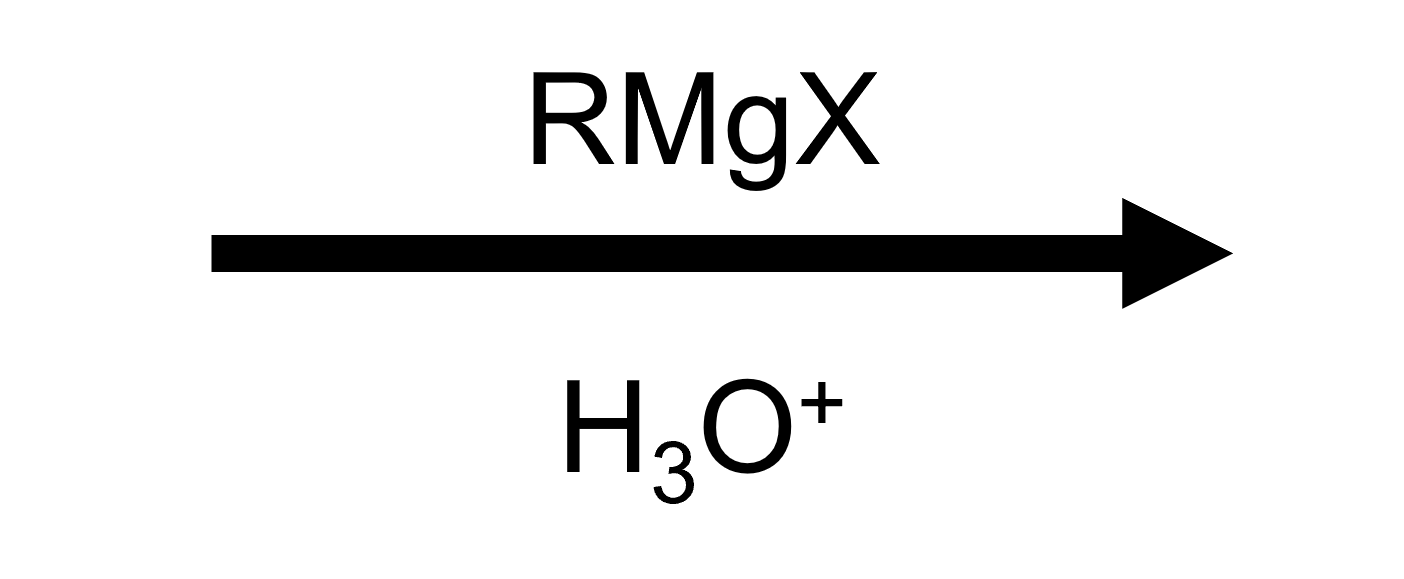
Product
Exam-Style Summary
RMgBr (dry Et₂O/THF, inert) adds R⁻ to the carbonyl carbon (1,2‑addition) to form a magnesium alkoxide. After the addition is complete, H₃O⁺ protonates the alkoxide → alcohol. Outcomes are diagnostic: formaldehyde → 1° ROH, aldehydes → 2° ROH, ketones → 3° ROH. No rearrangements occur (closed-shell pathway), moisture instantly kills the reagent, and new stereocenters are racemic unless chiral induction is specified.
Related Reading
- Alkyl Halide Reactions: Grignard Formation with Magnesium (RMgX) — how RMgBr reagents are prepared and why dry, inert technique matters.
- Conjugate (1,4‑) Addition to α,β‑Unsaturated Carbonyls with Gilman Reagents (R₂CuLi) — contrast hard (RMgBr, 1,2) vs soft (R₂CuLi, 1,4) nucleophiles.
Interactive Toolbox
- Mechanism Solver — choose the RMgBr button to replay the 3-step 1,2‑addition and H₃O⁺ workup.
- Reaction Solver — select a carbonyl and RMgBr to see the predicted alcohol class.
- IUPAC Namer — confirm names like 2‑pentanol or 1‑phenylcyclohexan‑1‑ol.
FAQ
What product do I get from RMgBr + aldehyde/ketone after H₃O⁺? Formaldehyde gives primary alcohols, other aldehydes give secondary alcohols, and ketones give tertiary alcohols—because the R group from RMgBr becomes the new substituent on the carbonyl carbon.
Why must the reaction be anhydrous? Water, alcohols, carboxylic acids, or even CO₂ protonate or react with RMgBr, destroying the reagent before it can add to the carbonyl.
Does the addition rearrange? No. The mechanism is closed-shell via a tetrahedral alkoxide; there are no carbocations, so hydride/methyl shifts do not occur.
Will RMgBr add 1,4 to an enone? Grignards are “hard” nucleophiles, so they favor 1,2‑addition even on α,β‑unsaturated carbonyls. Use R₂CuLi (Gilman) if you specifically need conjugate (1,4‑) addition.
What happens if I add RMgBr to an ester or acid chloride? Esters and acyl chlorides undergo multiple additions with RMgBr, typically ending at a tertiary alcohol (via a ketone intermediate). That is a different reaction manifold.
Are the products chiral? If the addition creates a stereocenter, the product is racemic under achiral conditions because attack occurs from either face of the planar carbonyl.