Aldehyde/Ketone → Alcohol with H₂ over Pd, Pt, or Ni
H₂ with a heterogeneous metal catalyst (Pd/C, Pt/C or PtO₂, Raney Ni, Rh/C) adds across C=O to give primary (aldehyde) or secondary (ketone) alcohols. The hydrogenation follows a surface-mediated Horiuti–Polanyi pathway: H₂ dissociates on the metal to surface hydrides, the carbonyl adsorbs, and two hydrogens are delivered sequentially to the carbonyl carbon and oxygen. Aldehydes typically reduce faster than ketones. In α,β-unsaturated systems, C=C reduction usually outpaces C=O with Pd or Ni, while Pt more readily reduces both. Mild conditions avoid arene saturation but hydrogenolysis of benzyl/allyl groups or nitro reduction can occur.
Quick Summary
- Reagents/conditions: H₂ (balloon → a few atm), Pt/C or PtO₂ (Adams’), Pd/C, Raney Ni; solvents EtOH, MeOH, EtOAc, THF; 0–60 °C.
- Outcome: Aldehyde → primary alcohol; ketone → secondary alcohol.
- Chemoselectivity: α,β-Unsaturated carbonyls hydrogenate at C=C faster than C=O with Pd/Ni; Pt reduces both. Aromatic rings usually survive mild conditions.
- Cautions: Pd/Ni can hydrogenolyze benzyl/allyl groups or reduce nitro; free thiols or coordinating amines poison catalysts.
- Mechanism: Surface H₂ dissociation → hydride to carbon → proton/hydride to oxygen → desorption (no radicals/carbocations).
Mechanism (4 Frames)
Worked Examples
Benzaldehyde → Benzyl Alcohol
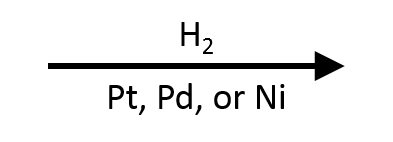
Benzaldehyde + H₂/Pd-C (EtOH, rt) → benzyl alcohol. Fast, chemoselective carbonyl reduction.
Cyclohexanone → Cyclohexanol
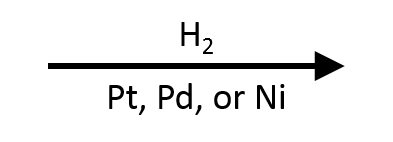
Cyclohexanone + H₂/Raney Ni (EtOH, 30 °C) → racemic cyclohexanol.
Cinnamaldehyde (Chemoselectivity)
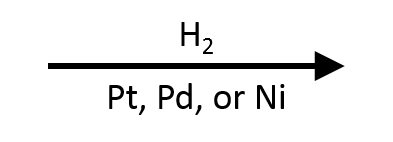
Cinnamaldehyde + H₂/Pd-C (balloon, rt) → hydrocinnamaldehyde (C=C reduced first). Prolonged exposure → 3-phenyl-1-propanol (use non-H₂ methods for allylic alcohol).
Scope & Limitations
- Great: Aliphatic/benzylic aldehydes and ketones; cyclic ketones; α-halo carbonyls (watch for hydrogenolysis of benzylic halides).
- Slower: Hindered dialkyl ketones; substrates with strongly coordinating heteroatoms (pyridyl, thioethers) unless catalyst tuned.
- Caution: Benzyl/allylic C–O and C–X groups cleave on Pd; nitro groups reduce to amines; S-containing compounds poison catalysts (Raney Ni cleaves thioacetals).
- Chemoselectivity knobs: Catalyst choice (Pd vs Pt vs Ni), H₂ pressure, temperature, and solvent polarity. Pd/Ni favor C=C hydrogenation first; PtO₂ hits both more rapidly.
- Labeling: Using D₂ installs D at carbon and oxygen (common exam question).
Practical Tips & Pitfalls
- Degas solvent and keep Pd/C or Raney Ni wet; dry Pd/C can ignite on exposure to air.
- Start with rt and balloon pressure; raise temperature or pressure only if conversion stalls.
- Filter catalysts through celite before concentrating; avoid metal fines in rotavap flasks.
- Decide upfront whether you want the C=C intact (use NaBH₄/Luche) or fully saturated (use Pd/Ni).
- Protect benzyl/allylic groups if hydrogenolysis would be problematic.
Exam-Style Summary
H₂ with Pd, Pt, or Ni adds across C=O on a metal surface, delivering hydride to carbon then proton to oxygen. Aldehydes → 1° alcohols, ketones → 2° alcohols, racemic when new stereocenters form. In α,β-unsaturated systems, C=C usually hydrogenates before C=O (Pd/Ni). Watch for catalyst poisoning (S/N donors) and hydrogenolysis of benzyl/allyl groups.
Interactive Toolbox
- Mechanism Solver — View H₂ dissociation, hydride delivery, protonation, and desorption; toggle catalyst icons to highlight chemoselectivity.
- Reaction Solver — Choose substrate class and catalyst to preview C=C vs C=O reduction priority and hydrogenolysis risks.
- IUPAC Namer — Confirm names for the resulting primary/secondary alcohols (no raw SMILES shown).