Carbonyl Reactions: Keto–Enol Tautomerization with H₃O⁺
Keto–enol tautomerization in acidic water begins with protonation of the carbonyl oxygen, followed by removal of an α-hydrogen. The resulting enol is neutral and quickly reverses: protonation at the alkene carbon and deprotonation of oxygen regenerate the keto form and the hydronium catalyst. Simple aldehydes and ketones remain predominantly keto, yet conjugation, aryl substitution, and 1,3-dicarbonyl stabilization elevate enol character. Because the enol is planar, repeated tautomerization racemizes α-stereocenters and enables α-deuterium incorporation in D₂O/D₃O⁺.
Quick Summary
- Reagents/conditions: Dilute strong acid in water (H₃O⁺/H₂O), typically 0–25 °C.
- Mechanism: Protonate the carbonyl oxygen, remove the α-hydrogen to give the enol, then protonate the enol C=C and deprotonate oxygen to reform the carbonyl.
- Equilibrium: Keto ≫ enol for simple carbonyls; enol content rises with conjugation, aryl substitution, and 1,3-dicarbonyl stabilization (resonance + intramolecular H-bonding).
- Carbon selection: Always pull the α-H from the more substituted carbon (fewer hydrogens) to capture the correct, conjugated enol orientation.
- Stereochemical outcome: α-Stereocenters racemize through the planar enol intermediate; repeated cycling in D₂O swaps α-H for α-D.
- Uses: Pre-equilibrium for electrophilic α-functionalization, isotope labeling, and pathways that capture enols under acidic conditions (e.g., halogenation).
Mechanism (Acid-Catalyzed Enolization)
Worked Examples
“Ketone Linear” template → most substituted enol
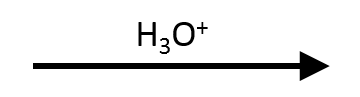
Water strips the α-hydrogen from the more substituted carbon, giving the internal alkene that our shared linear-ketone SMILES encodes.
Aldehyde template (butanal) → small enol fraction
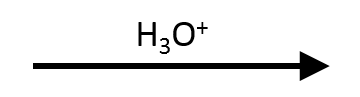
Simple aldehydes enolize quickly yet stay mostly keto—perfect for showing hidden α-H ⇌ α-D exchange in D₂O.
“Ketone Ring” template (cyclohexanone)
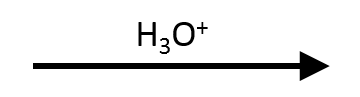
Cyclic ketones feed the “Ketone Ring” SMILES: the α-carbon becomes planar, erasing any stereochemistry adjacent to the carbonyl.
The remaining shared templates behave similarly:
- Benzaldehyde (our aromatic aldehyde SMILES) leverages ring conjugation to nudge the enol fraction upward, so α-electrophilic substitutions run quickly after tautomerization.
- Cinnamaldehyde (the conjugated alkene/aryl aldehyde SMILES used for crossover mechanisms) gains even more stabilization from the extended π-system; remind students that the drawn enol must retain that conjugation.
Pivaldehyde — no α-H, no enol
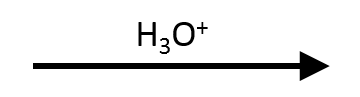
Tert-alkyl aldehydes (e.g., pivaldehyde) have no α hydrogens; acid only protonates the carbonyl—no tautomerization occurs.
Benzophenone — aromatic α-carbon lacks H
Carbonyls attached directly to sp² carbons (e.g., benzophenone) lack α-H and stay keto under acid; look for alternative activation.
Scope & Limitations
- Keto-favored: Simple aldehydes/ketones, where the carbonyl is typically 5–15 kcal·mol⁻¹ lower in energy than the enol.
- Enol-favored: β-Dicarbonyls, β-keto esters, phenacyl derivatives, and substrates with intramolecular hydrogen bonding or extended conjugation.
- Functional groups: Basic sites (amines) are protonated and may quench acid; strongly electrophilic media can trap the enol (e.g., halogenation). Aldehydes risk hydration/acetalization under harsher conditions.
- Solvent & acid strength: Use catalytic amounts of strong acid in water. Stronger acid or non-aqueous media can promote side reactions or polymerization of reactive enols.
Practical Tips & Pitfalls
- Use D₂O/D₃O⁺ to demonstrate α-deuterium incorporation; multiple cycles are required for full exchange.
- To observe low-enol populations, employ low-temperature NMR, IR signatures of O–H, or trap the enol with electrophiles under controlled conditions.
- Guard against racemization of valuable α-stereocenters; neutral or basic conditions may be preferable when chirality must be conserved.
- Avoid invoking enolates under acid—acidic media furnish neutral enols, whereas enolates belong to base-catalyzed manifolds.
Exam-Style Summary
H₃O⁺ protonates the carbonyl oxygen, water removes the α-hydrogen to form the enol, and the reverse (C-protonation followed by O-deprotonation) restores the keto tautomer. The equilibrium is usually keto-favored but shifts toward the enol with conjugation or 1,3-dicarbonyl stabilization. Repeated cycling racemizes α-centers and enables α-D exchange in D₂O/D₃O⁺.
Related Reading
- Ketone enol halogenation (acid)
- Ketone α-alkylation (strong base)
- Haloform reaction of methyl ketones
Interactive Toolbox
- Mechanism Solver — Animate protonation → α-deprotonation → enol formation → reverse keto regeneration; toggle β-dicarbonyl scenarios to visualize enol bias.
- Reaction Solver — Compare keto/enol fractions, racemization risks, and isotope-labeling notes for aldehydes, ketones, and β-dicarbonyls under acid.
- IUPAC Namer — Confirm names for keto versus enol tautomers without exposing structural encodings.