Alkyne Reactions: Ozonolysis to Carboxylic Acids (O₃ / H₂O)
Passing ozone through an aqueous alkyne mixture cleaves the C≡C bond to yield carboxylic acids. Each triple-bond carbon is oxidized all the way to the carboxylate oxidation state; when the alkyne is terminal, the vinylic carbon bearing hydrogen is pushed further to CO₂. The sequence parallels classic alkene ozonolysis, but internal alkynes stop only once both fragments reach their highest practical oxidation levels. For a refresher on alkene ozonolysis variants, compare the O₃ / DMS (reductive) and O₃ / H₂O₂ (oxidative) guides.
This walkthrough highlights the Criegee pair formed from an alkyne, shows how aqueous oxidation funnels products to acids (or CO₂), and provides strategy notes for terminal vs internal substrates. The worked examples mirror the SMILES used in our mechanism regression tests so you can move seamlessly between solver output and study notes.
Quick Summary
- Reagents/conditions: O₃ bubbled through an aqueous medium (0 to 25 °C). Maintain excess ozone or include H₂O₂ to drive complete oxidation.
- Outcome: Internal alkynes (R–C≡C–R′) cleave to RCO₂H + R′CO₂H. Terminal alkynes deliver RCO₂H and CO₂.
- Mechanism: 1,3-dipolar cycloaddition → molozonide → fragmentation to a Criegee carbonyl oxide → hydrolysis/oxidation to carboxylic acids.
- Stereochemistry: No stereochemical products remain once cleavage occurs; focus instead on oxidation state.
- Selectivity: Other oxidisable groups (allylic, benzylic, sulfur, amine) may over-oxidize. Control exposure time for sensitive motifs.
- Contrast: Reductive alkene ozonolysis (O₃ / DMS) stops at carbonyls; oxidative alkene ozonolysis (O₃ / H₂O₂) parallels this alkyne workflow.
Mechanism (4 Frames) (Criegee logic applied to alkynes)
Showcased substrate: but-2-yne → 2 equivalents of acetic acid.
The alkyne π electrons attack the electrophilic terminal oxygen while the O–O bond reorganises. The product is a cyclic peroxide that immediately sets up fragmentation.
Bond reorganisation yields a zwitterionic carbonyl oxide adjacent to a carbonyl fragment. Each carbon from the original alkyne now sits in its own electrophilic environment, primed for hydration and further oxidation.
In aqueous media the carbonyl oxide is rapidly intercepted by water and in-situ peroxide to form hydroperoxy intermediates. These species rearrange and eliminate to push each carbon toward the carboxylic acid oxidation state.
The reaction culminates in free carboxylic acids (or their conjugate bases). Terminal alkynes form one acid plus carbon dioxide; internal alkynes yield two acids whose carbon chains mirror the original substituents.
Mechanistic Checklist
- Track ozone as a 1,3-dipole. The alkyne behaves similarly to an alkene, but the higher oxidation state of the carbons encourages full cleavage.
- Expect a carbonyl oxide + carbonyl pair before hydrolysis. These are the Criegee intermediates that dictate downstream oxidation.
- Aqueous media supply both nucleophiles (H₂O) and oxidants (H₂O₂ generated in situ). This drives products to acids.
- Terminal alkynes: one fragment carries substituents and ends as RCO₂H; the unsubstituted carbon becomes CO₂.
- Compare to alkene ozonolysis: the difference lies in oxidation level, not initial cycloaddition. See the alkene-focused guides for reductive vs oxidative workups.
Worked Examples
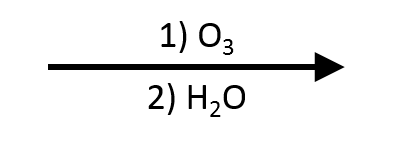
Compare to alkene O₃ / H₂O₂ for the alkene analogue.
Symmetrical internal alkyne → two identical carboxylic acids.
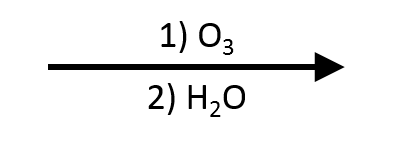
Each carbonyl carbon becomes propionic acid.
Longer chains give matching carboxylic acids; chain length does not affect outcome.
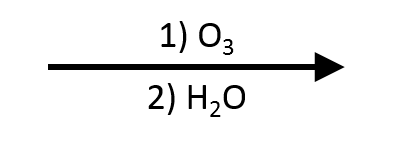
Terminal carbon fully oxidizes to CO₂.
Terminal alkyne → one carboxylic acid and gaseous CO₂.
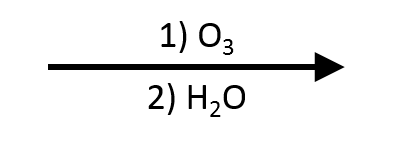
Branched fragments remain intact; the alkyne carbon is cleaved.
Branched substituents remain intact; only the atoms of the triple bond are cleaved.
Multiple Unsaturations & Selectivity
- Alkynes are cleaved preferentially, but isolated alkenes can also ozonise. Protect or sequence your reactions if multiple π bonds are present.
- Aromatic rings generally survive under cold, controlled ozonation but longer exposure can oxidize them. The O₃ / DMS alkene guide discusses approaches when you need to preserve aromatics.
- Dialkynes or enynes can generate mixed products. Dose ozone carefully, monitor reaction progress, and quench once the desired fragment pattern appears.
- Seeking aldehydes or ketones instead of acids? Switch to a reductive workup, as detailed in the alkene ozonolysis guides, before aqueous oxidation occurs.
Practical Tips & Pitfalls
- Temperature control: Run at −78 to 0 °C to limit ozonide buildup and side oxidation.
- Quench strategy: Purge residual ozone, then add your chosen oxidant (H₂O₂) or reductant. Never warm an ozonide-laden solution without first quenching.
- Gas handling: Ozone is toxic and highly oxidising. Work in a fume hood with an ozone destruct unit and resistant tubing.
- Terminal alkynes: Expect CO₂ evolution. Vent carefully and use traps if capturing gases for analysis.
- Sensitive substituents: Sulfides, tertiary amines, and electron-rich aromatics may over-oxidize. Protect them or consider alternative cleavage reagents.
Exam-Style Summary
O₃ / H₂O converts alkynes into carboxylic acids via cycloaddition, Criegee fragmentation, and oxidative hydrolysis. Internal alkynes yield two acids; terminal alkynes give one acid plus CO₂. Relate the sequence to alkene ozonolysis to predict products rapidly on exams or in synthesis planning.
Interactive Toolbox
- Mechanism Solver — explore the alkyne ozonolysis mechanism (
alkyneO3MechanismFunction) step by step. - Reaction Solver — contrast alkyne ozonolysis with alkene ozonolysis or permanganate cleavage.
- IUPAC Namer — practice naming carboxylic acids such as acetic, propionic, benzoic, and pivalic acid.
Related Guides