Wittig Reaction — Ph₃P Ylide → Alkene (Wittig Olefination)
Wittig Reaction — Alkene Formation from Aldehydes/Ketones via Ph₃P and Alkyl Halides
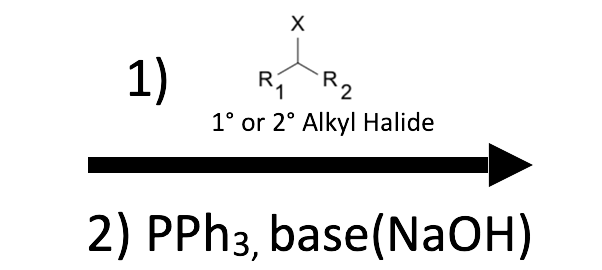
Triphenylphosphine (PPh₃) displaces halide from an SN2‑compatible alkyl halide to give a phosphonium salt. A base then removes the α‑hydrogen to generate the phosphonium ylide, which adds to an aldehyde or ketone, passing through an oxaphosphetane and collapsing to an alkene plus triphenylphosphine oxide (Ph₃P=O). Non‑stabilized ylides normally deliver Z‑alkenes; stabilized ylides (bearing EWG on the ylide carbon) favor E‑alkenes. Aldehydes react faster than ketones, and forming the ylide is usually the rate‑determining gate.
Introduction
- Two-stage logic: (1) Ylide generation — PPh₃ + primary/allylic/benzylic R–X → phosphonium salt; base → ylide. (2) Carbonyl coupling — ylide + aldehyde/ketone → oxaphosphetane → alkene + Ph₃P=O.
- Driving force: Formation of a strong P=O bond when the oxaphosphetane collapses.
- Halide scope: Primary (best), allylic, or benzylic halides give clean SN2. Unactivated secondary halides are unreliable; tertiary/vinyl/aryl halides fail.
- E/Z heuristic: Non‑stabilized ylides → Z‑alkenes. Stabilized ylides (ylide carbon bears EWG) → E‑alkenes.
- Substrate reactivity: Aldehydes > ketones. Hindered ketones may require heating or Horner–Wadsworth–Emmons alternatives.
Quick Summary
- Salt formation (SN2): PPh₃ + primary/allylic/benzylic R–X → R–PPh₃⁺ X⁻. Avoid unactivated secondary halides (SN2 too slow; elimination).
- Ylide generation: Strong base (n‑BuLi, NaHMDS, t‑BuOK) for non‑stabilized salts; milder bases (NaH, NaOMe) suffice for stabilized salts.
- Carbonyl coupling: Add aldehyde/ketone in dry ether solvent (THF/Et₂O) at 0–25 °C → betaine/oxaphosphetane → alkene + Ph₃P=O.
- Stereochemistry rule-of-thumb: Non‑stabilized ylides → Z. Stabilized ylides → E. (Schlosser modification can invert the unstabilized case but is advanced.)
- Common pitfalls: Attempting to form salts from simple secondary halides, choosing a base that cannot deprotonate the salt, or forgetting that cyclic ketones deliver exocyclic alkenes.
Mechanism — Ylide Generation then Carbonyl Coupling
Part A — Ylide Generation (optional display in the Mechanism Solver)
Part B — Carbonyl Coupling
Mechanistic Checklist (Exam Focus)
- Confirm the alkyl halide partner is SN2‑compatible (primary or allylic/benzylic); do not draw SN2 at a simple secondary/tertiary center.
- Match base strength to the ylide (strong base for non‑stabilized salts, milder base for stabilized salts).
- Depict the addition → betaine → oxaphosphetane → alkene + Ph₃P=O sequence with closed-shell arrows.
- Predict Z for non‑stabilized ylides, E for stabilized ylides (unless the prompt specifies Schlosser conditions).
- For cyclic ketones, show exocyclic alkenes (the new double bond leaves the ring).
Worked Examples
Reactant
Benzaldehyde (aldehyde partner)
Reagents
Seafoam-teal Color 1 fragment = terminal =CH₂ unit from methyl bromide
Product
Styrene (terminal =CH₂ carbon from the methylene ylide is seafoam-teal Color 1)
Reactant
Cyclohexanone (cyclic ketone)
Reagents

Seafoam-teal Color 1 fragment = benzylidene portion delivered by benzyl bromide
Product
Benzylidenecyclohexane (benzyl ring + benzylic carbon from the ylide = seafoam-teal Color 1)
Scope & Limitations
- Best halides for salt formation: Primary > allylic ≈ benzylic ≫ simple secondary (poor). Tertiary/vinyl/aryl halides fail (no SN2).
- Carbonyl partners: Aldehydes are fast; ketones may need heat or different ylides. Conjugated carbonyls work but may give blends.
- Ylide classes: Non‑stabilized (alkyl/benzyl) require strong bases and typically give Z. Stabilized ylides (CO₂R, COR, CN, SO₂R) are easier to form and give E.
- Selectivity tweaks: Schlosser modifications (n‑BuLi then strong alkoxide at low T) can invert the non‑stabilized case to E, but this is beyond the introductory scope.
Practical Tips & Pitfalls
- Form the phosphonium salt cleanly (heat/polar solvents help) before you add strong base.
- Keep all ylide steps dry and under inert atmosphere; moisture/protic solvents quench both base and ylide.
- Choose the base based on ylide acidity: n‑BuLi/NaHMDS for non‑stabilized salts, NaH/NaOMe for stabilized salts.
- Expect triphenylphosphine oxide as a stoichiometric byproduct; plan for its removal (crystallization, silica plug).
- If you truly need an E‑alkene from a hindered ketone, consider Horner–Wadsworth–Emmons or Julia–Kocienski routes instead.
Exam-Style Summary
PPh₃ + primary/allylic/benzylic alkyl halide → phosphonium salt; strong/mild base → ylide; ylide + aldehyde/ketone → oxaphosphetane → alkene + Ph₃P=O. Unstabilized ylides give Z (major), stabilized ylides give E (major), and simple secondary halides are not reliable ylide precursors.
Related Reading
- Carbonyl → alcohol via Grignard (RMgBr)
- Carbonyl reduction (LiAlH₄)
- Ketone α-alkylation (strong base)
Interactive Toolbox
- Mechanism Solver — Toggle halide class (primary vs allylic/benzylic vs blocked secondary), ylide type (non‑stabilized vs stabilized), and carbonyl to see every RDKit-rendered frame.
- Reaction Solver — Compare predicted outcomes for different carbonyls or ylides and log any warnings about the halide input.
- IUPAC Namer — Confirm systematic names of the alkene products shown in the worked examples (no SMILES exposed to users).
FAQ
Which alkyl halides can I use to make the phosphonium salt?
Primary, allylic, and benzylic halides work well through SN2. Simple secondary halides usually give low conversion or elimination, and tertiary/vinyl/aryl halides fail.
How do I choose the base for ylide formation?
Match it to the salt: non‑stabilized salts need strong bases (n‑BuLi, NaHMDS, t‑BuOK), whereas stabilized salts (with EWG) can be deprotonated by NaH/NaOMe.
How do I predict the alkene geometry?
Use the intro rule: non‑stabilized ylides → Z, stabilized ylides → E. Aldehydes give cleaner selectivity than ketones. Schlosser conditions can invert the unstabilized case but are beyond this course.
Do ketones behave the same as aldehydes?
They’re slower and more sterically hindered. Exocyclic alkenes form when cyclic ketones are used.
Where does the oxygen go?
Into the triphenylphosphine oxide byproduct (Ph₃P=O). The alkene product contains only the carbon framework from the carbonyl carbon and the ylide carbon.