Acid Chloride to Amide (NH3, RNH2, R2NH)
Exam Answer
- Reagents
- NH3 or amines (often with base)
- Major outcome
- RCOCl to amide (RCONH2 / RCONHR / RCONR2)
- Selectivity
- Fast nucleophilic acyl substitution; moisture-sensitive starting material
Common traps
- Amines often used in excess to suppress over-acylation side products
- Water contamination gives carboxylic acid (or salt)
- Tertiary amines can act as base but cannot form amides
Acid chlorides acylate amines rapidly to give amides via nucleophilic acyl substitution. Using NH₃, RNH₂, or R₂NH yields primary, secondary, or tertiary amides, respectively. Each acylation generates HCl, so you run with excess amine (nucleophile + base) or with an added base/HCl scavenger (pyridine, Et₃N, or aqueous NaHCO₃/Na₂CO₃ under Schotten–Baumann biphasic conditions).
Quick Summary
Reagents/conditions: Acid chloride + amine (2 eq) or 1 eq amine + base (1–2 eq); 0 °C → rt; dry solvent or Schotten–Baumann (biphasic base).
Outcome: NH₃ → 1° amide; RNH₂ → 2° amide; R₂NH → 3° amide.
Notes: Base/excess amine traps HCl (ammonium/pyridinium/Et₃NH⁺Cl⁻); keeps nucleophile free.
Mechanism — 4 Steps (Closed-Shell Nucleophilic Acyl Substitution)
- Amine attack (tetrahedral intermediate): Amine lone pair (NH₃/RNH₂/R₂NH) attacks the acyl carbon; C=O π shifts to oxygen → O⁻/N⁺ tetrahedral adduct.
- Collapse; chloride leaves: O⁻ re-forms C=O; Cl⁻ departs to give an N‑acylammonium (protonated amide).
- Deprotonation to neutral amide: Base (excess amine, pyridine, Et₃N, or carbonate) removes N–H, yielding the neutral amide and ammonium/pyridinium chloride.
- Product frame: Amide product (primary/secondary/tertiary) with the corresponding ammonium/pyridinium/Et₃NH⁺Cl⁻ salt.
Mechanistic Checklist (Exam Focus)
- Show tetrahedral addition → collapse; Cl⁻ is the leaving group.
- Include deprotonation/base step; otherwise amine becomes RNH₃⁺Cl⁻ and stalls.
- Map outcomes by amine class: NH₃ → 1°, RNH₂ → 2°, R₂NH → 3° amide; 3° amines act only as base.
- Moisture competes (hydrolysis); Schotten–Baumann uses basic water to neutralize HCl continuously.
- Anilines are slower; still acylate due to acid chloride reactivity—use base/longer time.
Worked Examples
Reactant
Reagent

NH₃; excess traps HCl.
Product
Benzamide + NH₄Cl (not shown)
Reactant
Reagent
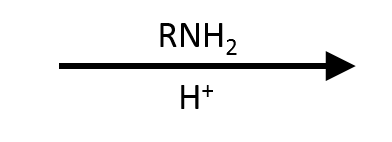
EtNH₂ (2 eq or + base)
Product
Secondary amide
Reactant
Reagent
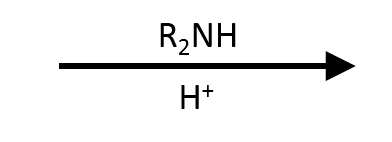
di-n-propylamine + base
Product
Tertiary amide (salt byproduct not shown)
Scope & Limitations
- Works well: Aliphatic/aromatic acid chlorides with NH₃, primary, or secondary amines. Anilines react but may need stronger base/longer time.
- Amines as base: 2 eq amine (one nucleophile, one base) is standard; external bases (pyridine/Et₃N/carbonate) keep amine deprotonated.
- Schotten–Baumann: Biphasic H₂O/organic with NaHCO₃/Na₂CO₃ neutralizes HCl as it forms.
- Tertiary amines: Bases only (no N–H); they do not furnish amides.
- Competing hydrolysis: Water without base yields carboxylic acid (hydrolysis). Keep dry unless intentionally biphasic/basic.
Practical Tips
- Add acid chloride slowly to cold amine/base to control exotherm and keep nucleophile free.
- Choose base by goal: excess amine (simple), pyridine (traps HCl, can catalyze), Et₃N (non-nucleophilic), carbonate buffer (Schotten–Baumann).
- For anilines/poor nucleophiles, avoid aqueous media unless using strong base; consider DMAP or heat if needed.
- Filter or extract salts (RNH₃⁺Cl⁻, PyH⁺Cl⁻, Et₃NH⁺Cl⁻) during workup; wash organic layer with dilute base.
Exam-Style Summary
RCOCl + NH₃/RNH₂/R₂NH → tetrahedral intermediate → collapse (Cl⁻ leaves) → base deprotonates → RCONH₂/RCONHR/RCONR₂. Use 2 eq amine or amine + base to trap HCl; tertiary amines are bases only.
FAQ
How do I drive acid chloride → amide instead of hydrolysis?
Exclude water or run Schotten–Baumann with base to neutralize HCl; keep amine deprotonated.
Can tertiary amines make amides?
No. They lack N–H; they only act as bases (Et₃N, pyridine).
Why use excess amine?
One equivalent acylates; the second scavenges HCl to maintain nucleophilicity.
Do anilines react?
Yes, but slower; use base/longer time. Acid chlorides are reactive enough to acylate anilines.
Interactive Toolbox
- Mechanism Solver — animate attack → collapse → deprotonation; toggle amine class and base mode (excess amine vs pyridine/Et₃N vs Schotten–Baumann).
- Reaction Solver — choose acid chloride and amine class to preview amide class and salt byproduct.
- IUPAC Namer — caption amide products without exposing SMILES.
Related Reading
- Acid chloride hydrolysis (H₂O, pyridine) — contrast amidation vs hydrolysis.
- Carbonyl → imine/enamine (amine condensation) — parallel amine addition on aldehydes/ketones.
- LiAlH₄ carbonyl reduction — related hydride pathway without leaving groups.