Gilman (R₂CuLi) 1,4-Addition to α,β-Unsaturated Carbonyls
Gilman (R₂CuLi) 1,4‑Addition to α,β‑Unsaturated Carbonyls
Lithium diorganocuprates (Gilman reagents, R₂CuLi) are soft carbon nucleophiles that selectively add at the β‑position of α,β‑unsaturated carbonyls (enones, enals, enoates). The reaction proceeds through conjugate (1,4‑) addition to generate a metal enolate, which is then protonated (to a saturated carbonyl) or trapped (e.g., alkylation, silylation). In contrast, harder nucleophiles like RMgX or RLi favor 1,2‑addition to the carbonyl carbon under similar conditions.
Quick Summary
- Reagents/conditions: R₂CuLi (from R–Li + CuI), dry Et₂O/THF, −78 °C → 0 °C; then H₃O⁺ workup (or electrophile trap).
- Outcome: β‑substituted ketone/aldehyde/ester (overall saturation of the C=C).
- Selectivity: R₂CuLi → 1,4‑addition; RMgX/RLi → 1,2‑addition (general rule).
- Enolate fate: Protonate (default) or trap (e.g., MeI for α‑alkylation; TMSCl for silyl enol ether).
- Scope: Works with enones, enals, enoates, and other activated Michael acceptors.
Mechanism — Conjugate Addition → Enolate → Workup (4 Steps)
Step 1 — Association and conjugate attack (1,4‑addition)
Step 2 — Enolate formation after conjugate transfer
Step 3 — Default workup: protonation
Step 3B — Electrophile trapping before protonation (optional)
Step 4 — Final workup panel
Mechanistic Checklist (Exam Focus)
- Soft vs hard nucleophiles: Cuprates are soft → 1,4 attack. Grignard/RLi are hard → 1,2 addition.
- Two‑stage logic: Conjugate transfer → enolate → workup (protonate or trap) defines the isolated product.
- Stereochemical notes: Addition creates/sets β‑stereocenters; enolate geometry and protonation face can bias diastereoselectivity in cyclic systems.
- Compatibility: Cuprates are less basic than RLi/RMgX, reducing side reactions (e.g., fewer eliminations).
- Reagent preparation: 2 R–Li + CuI → R₂CuLi (use immediately; air/moisture sensitive).
Worked Examples
Reactant
Reagent
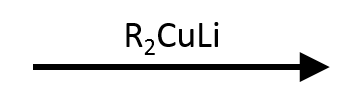
Product
Reactant
Reagent
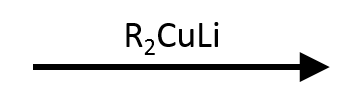
Product
Reactant
Reagent(s)
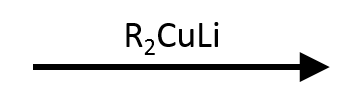
Product
Scope & Limitations
- Works well: Primary/secondary alkyl cuprates; aryl/vinyl cuprates also employed. Enones/enals/enoates are robust acceptors.
- Caution: Very electron‑poor substrates (multiple EWGs) can be over‑reactive; carefully control temperature.
- Not this manifold: For direct 1,2‑addition to simple aldehydes/ketones, use RMgX/RLi. For acyl substitution to ketones, use R₂CuLi with acid chlorides (separate topic).
- Functional groups: Cuprates tolerate many groups better than RLi/RMgX, but protic sites still quench the reagent; use anhydrous conditions.
Edge Cases & Exam Traps
- Misassigned regiochemistry: Drawing the R at the carbonyl carbon is wrong for cuprates—β‑carbon is attacked.
- Forgetting enolate stage: The C=C becomes saturated; the immediate product is an enolate, not a dihydro adduct with O‑H.
- Wrong reagent for goal: If you need 1,2‑addition, do not use R₂CuLi; choose RMgX/RLi.
- Overlooking trap options: Stopping at protonation is fine, but electrophile trapping is often tested as a strategic extension.
- Temperature control: Warming too early can lower selectivity or cause side processes.
Practical Tips
- Prepare fresh R₂CuLi at low temperature (−78 °C to 0 °C) in Et₂O/THF; exclude air/moisture.
- Add the Michael acceptor to the cuprate slowly to manage exotherm and maintain selectivity.
- For trapping, add the electrophile at low temperature before aqueous workup; then quench.
- Workup: Cold NH₄Cl/H₂O or dilute H₃O⁺; extract, dry, and purify.
Exam-Style Summary
R₂CuLi (soft) + α,β‑unsaturated carbonyl → 1,4‑addition (β‑attack) → metal enolate → H₃O⁺ (β‑substituted carbonyl) or trap (e.g., MeI/TMSCl). Use cuprates for 1,4‑selectivity; use RMgX/RLi for 1,2‑addition.
Related Reading
- Acid Chloride Reactions: Ketone formation using Gilman Reagents — contrasts 1,4‑addition to α,β‑unsaturated carbonyls with nucleophilic acyl substitution on acid chlorides.
- Carboxylic Acid Reactions: Acid Chloride formed using Carboxylic acids and SOCl₂ — pairs well when reviewing how acyl chlorides are prepared before cuprate additions.
Interactive Toolbox
- Mechanism Solver — choose the R₂CuLi button to watch the RDKit-rendered conjugate addition steps.
- Reaction Solver — plug in a substrate and select R₂CuLi to preview the expected β‑addition outcome.
- IUPAC Namer — generate names for the reactants or products shown in the worked examples.
FAQ
What is the Gilman reagent and why does it favor 1,4‑addition? A Gilman reagent is R₂CuLi, a soft organocuprate nucleophile. Soft nucleophiles interact preferentially with the softer β‑carbon of an α,β‑unsaturated carbonyl, giving conjugate (1,4‑) addition, whereas harder reagents (RMgX, RLi) add 1,2 to the carbonyl carbon.
Which substrates undergo 1,4‑addition with R₂CuLi? α,β‑Unsaturated carbonyls: enones, enals, and enoates (and other Michael acceptors). The reaction forms a β‑substituted carbonyl after enolate workup.
How do I choose between cuprate (R₂CuLi) and Grignard/RLi for a carbonyl addition? Use R₂CuLi for conjugate 1,4‑addition to unsaturated carbonyls. Use RMgX/RLi for direct 1,2‑addition to simple aldehydes/ketones (no conjugation).
What happens to the enolate intermediate—protonate or trap? Default is protonation with H₃O⁺ to give the saturated carbonyl. Alternatively, trap the enolate with an electrophile (e.g., MeI for α‑alkylation, TMSCl for silyl enol ether) before aqueous workup.
Do cuprates tolerate sensitive functional groups better than Grignards? Generally yes. Cuprates are less basic and more chemoselective, often minimizing elimination or over-reduction compared to RLi/RMgX; nevertheless, protic sites still quench them—keep conditions anhydrous.
Can I use R₂CuLi for making ketones from acid chlorides? Yes—acyl substitution of acid chlorides with cuprates affords ketones. That is a different manifold from the 1,4‑addition covered here and is typically treated separately.
Can I trap the enolate after 1,4‑addition? Yes. Add an electrophile (e.g., MeI) before the aqueous quench to obtain a conjugate addition–alkylation product (β‑R plus α‑electrophile).