Silyl Deprotection of Alcohols (TBAF, F⁻)
Fluoride-based deprotection (most commonly TBAF) converts a silyl ether (RO–SiR₃) back into the free alcohol (ROH). The key driving force is formation of a strong Si–F bond, so the chemistry happens at silicon, not at carbon.
Quick Summary
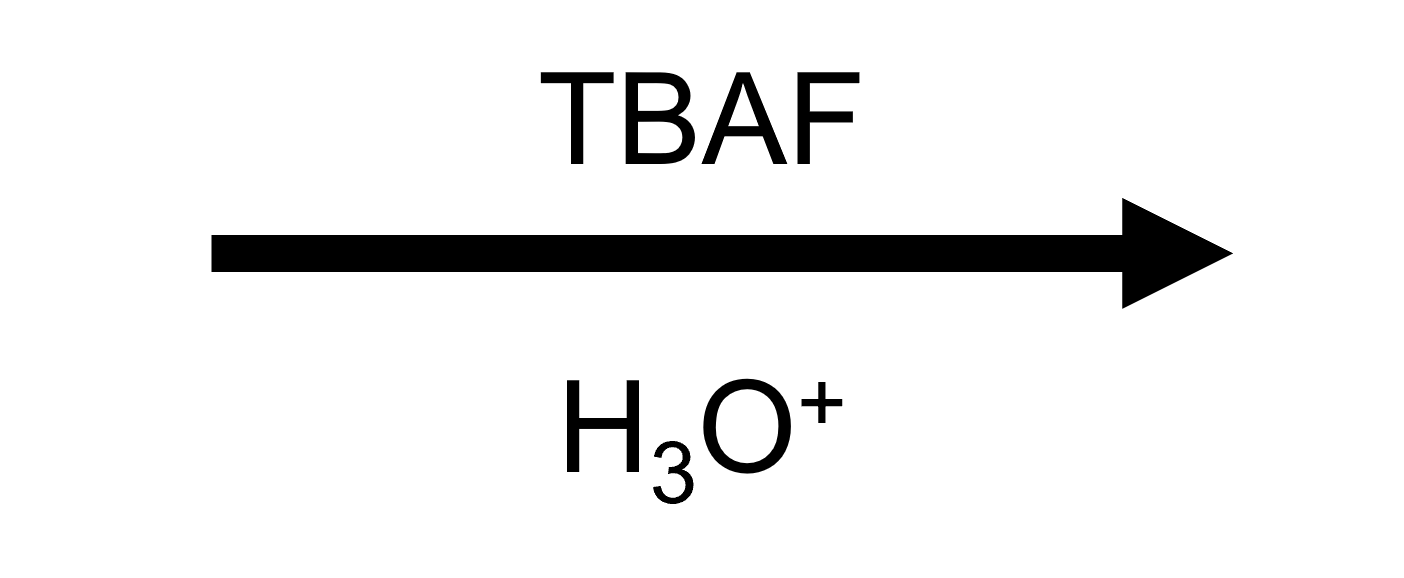
- Reagents/conditions: RO–SiR₃ + TBAF (F⁻) in THF, then aqueous workup.
- Outcome: RO–SiR₃ → ROH + R₃SiF.
- Mechanism: Two-step substitution at silicon: (1) F⁻ from TBAF attacks Si while Si–O bond breaks simultaneously, giving alkoxide (RO⁻) and silyl fluoride (R₃Si–F); (2) protonation during workup gives ROH.
- Stereochemistry: Retained at the alcohol carbon since substitution occurs at silicon, not carbon.
- Selectivity: TMS ethers are more labile (removed faster) than TBS ethers. Problems often test selective deprotection.
- Common pitfalls: Forgetting that without workup the product is RO⁻ (alkoxide), not ROH. Confusing which silyl group is removed first in molecules with multiple protections.
Mechanism — Two Steps + Product (TBAF Attack/Cleavage, Protonation, Product)
Each frame comes directly from the RDKit builder used in the Mechanism Solver. Substitution occurs at silicon, not carbon. The driving force is formation of the strong Si–F bond (~140 kcal/mol).
Mechanistic Checklist
- Substitution occurs at silicon, NOT at carbon — no SN1/SN2 at the alcohol carbon.
- No carbocation intermediates — no rearrangements possible.
- The driving force is Si–F bond formation (~140 kcal/mol bond energy vs ~90 kcal/mol for Si–O).
- Stereochemistry at the alcohol carbon is completely preserved.
- Without proton source/workup, the immediate product is RO⁻ (alkoxide), not ROH.
Worked Examples
Reactant
Reagent
Product
Ethanol.
Reactant
Reagent

Product
Cyclohexanol.
Reactant
Reagent

Product
Ethanol (TBS requires longer time than TMS).
Reactant
Reagent

Product
Isopropanol (secondary alcohol).
Reactant
Reagent

Product
tert-Butanol (tertiary alcohol).
Reactant
Reagent

Product
Ethanol (TIPS requires longer time than TBS).
Reactant
Reagent

Product
Ethanol (TBDPS most stable; may need extended time or heat).
Each worked example reuses the same SMILES that feed the Mechanism Solver, so what you see here is exactly what appears in the interactive tool.
Scope & Limitations
| Feature | TMS Ethers | TBS/TBDMS Ethers | TIPS Ethers | TBDPS Ethers |
|---|---|---|---|---|
| Fluoride lability | Very fast | Moderate | Slow | Very slow |
| Selective removal | Removed first | Survives when TMS is present | Survives when TMS/TBS present | Most robust |
| Typical conditions | TBAF, rt, minutes | TBAF, rt–40°C, hours | TBAF, 40–60°C, hours | TBAF, extended time/heat |
Rule of thumb (lability order): TMS > TBS > TIPS > TBDPS. In molecules with multiple silyl protecting groups, TMS is removed first, TBS second, TIPS third, and TBDPS survives the longest under fluoride conditions. This selectivity is frequently tested on exams.
Practical Tips
- Selective deprotection: Use controlled TBAF equivalents or short reaction times to remove TMS selectively while leaving TBS intact.
- Complete deprotection: Use excess TBAF and longer reaction times to remove all silyl groups.
- Alternative fluoride sources: HF·pyridine, TASF, and CsF can also deprotect silyl ethers; HF is more aggressive and can cleave TBS faster.
- Moisture sensitivity: TBAF solutions should be fresh; hydrated TBAF may be less effective.
- Workup matters: If the problem doesn't show aqueous workup, the product may be RO⁻ rather than ROH.
Exam-Style Summary
Silyl ether + TBAF (F⁻) → alcohol. The mechanism is two steps: (1) F⁻ attacks Si while Si–O breaks simultaneously (driven by strong Si–F bond formation), then (2) protonation gives ROH. Stereochemistry at carbon is retained. TMS is more labile than TBS — expect selective deprotection questions.
Pitfalls to watch for:
- Drawing SN2 at the alcohol carbon — substitution occurs at silicon, not carbon.
- Forgetting workup — without protonation, the product is RO⁻ (alkoxide), not ROH.
- Confusing lability order — TMS is removed faster than TBS.
- Missing selective deprotection — if both TMS and TBS are present, TMS comes off first.
FAQ
Why does fluoride remove silyl groups? Silicon has a strong affinity for fluorine (~140 kcal/mol Si–F bond vs ~90 kcal/mol Si–O). This thermodynamic driving force makes fluoride attack at silicon highly favorable.
What's the difference between TMS and TBS under fluoride? TMS ethers are smaller and more accessible, so they're cleaved faster. TBS has a bulky tert-butyl group that slows fluoride attack. In mixed systems, TMS is removed first.
What if the problem doesn't show workup? The immediate product of fluoride deprotection is the alkoxide (RO⁻), not the alcohol (ROH). Protonation happens during aqueous workup. Some problems test this distinction.
Can I deprotect TBS without touching TMS? No — TMS is more labile than TBS. If you treat a molecule with both, TMS is removed first. To selectively remove TBS, you'd need to first remove TMS (or use different conditions).
What about silyl enol ethers? TBAF can also cleave silyl enol ethers and other silyl-protected functionalities. Watch for unintended desilylation in complex molecules.
Interactive Toolbox
Use these tools to explore silyl deprotection:
- Mechanism Solver — see the two-step RDKit mechanism for TBAF deprotection on any silyl ether.
- Reaction Solver — enter your silyl ether, select TBAF, and confirm the alcohol product.
- IUPAC Namer — generate systematic names for your alcohol products.
Related Reading
- Alcohol Protection with TMSCl/TBSCl — the reverse reaction: protecting alcohols as silyl ethers.
- Williamson Ether Synthesis — making ethers via SN2 with alkoxide + alkyl halide.
- Alcohol Oxidation — converting alcohols to aldehydes, ketones, or carboxylic acids.